Epirubicin hydrochloride
-
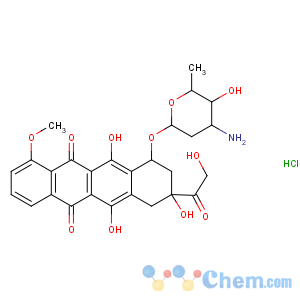
Molecular Structure
Detailed Description
Epirubicin hydrochloride
Synonyms:(8s-cis)-rochlorid;4’-epi-adriamycinhydrochloride;5,12-naphthacenedione,10-((3-amino-2,3,6-trideoxy-alpha-l-arabino-hexopyranosy;8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-l)oxy)-hyd;EpirubicinHc,LEp5,;Adrial;Ellence, Farmorubicin, Pharmorubicin
CAS:56390-09-1
MF:C27H30ClNO11
MW:579.9802
EINECS:260-145-2
Chemical Properties:Orange-Red Crystalline Solid
Usage:Used as an antineoplastic
Biological Activity:Antibiotic antitumor agent. Inhibits the synthesis and function of DNA (IC 50 = 62.7 μ M in rat glioblastoma cell lines) and inhibits the relaxing property of topoisomerase II.
TEST SPECIFICATION RESULT
Appearance Orange-red crystalline powder; No visible evidence of contamination by foreign matter. Conforms
Solubility Soluble in water and methanol; slightly soluble in ethanol; practically insoluble in acetone. Conforms
Identification A IR spectrum corresponds to the IR spectrum of the reference standard Conforms
Identification B The principle peak in the chromatogram obtained with the test solution under Assay is similar in retention time to the principle peak in the chromatogram obtained with the reference solution. Conforms
pH 4.0 to 5.5 5.0
Water Content Not More Than 4.0% 1.6%
Related Substances
Impurity A (Doxorubicinone) Not More Than 1.0% 0.2%
Impurity C (Doxorubicin) Not More Than1.0% 0.5%
Any Other Impurities Not More Than 0.5% 0.3%
Total Impurities Not More Than 2.0% 1.5%
Assay 97.0% to 102.0% of C27H30ClNO11 calculated on the anhydrous, solvent-free basis 98.2%
Residual Solvents
Acetone Not More Than 15000ppm 3200ppm
Ethanol Not More Than5000ppm 2050ppm
Ethyl Acetate Not More Than 5000ppm Not detected
Methanol Methylene Chloride Total Residual Solvents Not More Than 1000ppm Not More Than 500ppm Not More Than 20000ppm 100ppm Not detected 4350ppm
Conclussion To be complied with EP 7.0 specifications

- Epirubicin hydrochloride




