Idarubicin hydrochloride
-
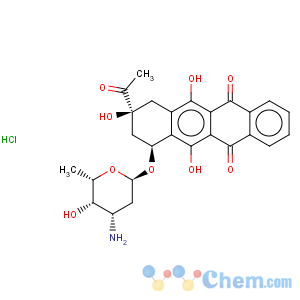
Molecular Structure
Detailed Description
Idarubicin hydrochloride
Synonyms:idarubicin hcl;idarubicin hydrochloride;4-demethoxy-daunomycihydrochloride;4-demethoxydaunorubicinhydrochloride;(7s-cis)-9-acetyl-7-[(3-amino-2,3,6-trideoxy-α-l-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-5,12-naphthacenedione;4-DMD HCl;5,12-Naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, hydrochloride, (7S,9S)-;5,12-Naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, hydrochloride, (7S-cis)-
CAS:57852-57-0
MF:C26H28ClNO9
MW:533.95
EINECS:260-990-7
Chemical Properties:Orange Solid
Usage:Idarubicin HCl is a hydrochloride salt form of Idarubicin which is an anthracycline antibiotic and a DNA topoisomerase II (topo II) inhibitor for MCF-7 cells with IC50 of 3.3 ng/mL.Orally active anthracycline; analog of Daunorubicin. Antineoplastic.
TEST SPECIFICATION RESULT
Appearance Orange-red crystalline powder; No visible evidence of contamination by foreign matter. Conforms
Solubility Soluble in water and methanol; slightly soluble in ethanol; practically insoluble in acetone. Conforms
Identification A The infrared spectrum exhibits maxima only at the same wavelengths as that of a similar preparation of the reference standard. Conforms
Identification B The chromatogram of the assay preparation obtained in the assay exhibits a major peak for Idarubicin, the retention time of which corresponds to that in the chromatogram of the standard preparation Conforms
pH 5.0 to 6.5 6.1
Water Content Not More Than 5.0% 3.6%
Chromatographic Purity
Individual Impurity Not More Than 0.2% 0.11%
Total Impurities Not More Than3.0% 0.7%
Assay 960 μg to 1030 μg per mg calculated on the anhydrous, solvent-free basis 988μg /mg
Residual Solvents
Ethanol Not More Than3000ppm 850ppm
Methanol Organic Volatile Impurities Methylene Chloride Dioxane Bacterial Endotoxins Not More Than 3000ppm Not More Than 600ppm Not More Than 380ppm Not More Than 8.9 EU/MG 600ppm Not detected Not detected <0.38EU/MG
Conclusion To be complied with USP 34 specifications
Ethisterone CAS : 434-03-7
Norethisterone CAS:68-22-4
Ethynyl estradiol CAS:57-63-6
Ethynodiol diacetate CAS:297-76-7
Chlormadinone acetate CAS.: 302-22-7
Cyproterone acetate CAS.: 427-51-0
Megestrol acetate CAS.: 595-33-5
Estradiol CAS:50-28-2
17alpha-Oestradiol CAS: 57-91-0
Estradiol Cypionate CAS:313-06-4
Estradiol enantate CAS: 4956-37-0
Estradiol valerate CAS: 979-32-8
Estradiol Benzoatae CAS:50-50-0
Estrone CAS: 53-16-7
Estriol CAS: 50-27-1
19-Norethindrone acetate CAS:51-98-9
Norethisterone enanthate CAS:3836-23-5
Testolactone CAS:968-93-4
Eplerenone CAS: 107724-20-9
Progesterone CAS:57-83-0
Medroxyprogesterone Acetate CAS: 71-58-9

- Idarubicin hydrochloride




