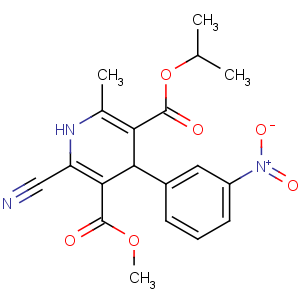
Title: Nilvadipine
CAS Registry Number: 75530-68-6
CAS Name: 2-Cyano-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-methyl 5-(1-methylethyl) ester
Synonyms: 5-isopropyl-3-methyl-2-cyano-1,4-dihydro-6-methyl-4-(
m-nitrophenyl)-3,5-pyridinedicarboxylate; isopropyl 6-cyano-5-methoxycarbonyl-2-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylate; nivadipine; nivaldipine
Manufacturers' Codes: FR-34235; FK-235; SKF-102362
Trademarks: Escor (Merck KGaA); Nivadil (Fujisawa)
Molecular Formula: C19H19N3O6
Molecular Weight: 385.37
Percent Composition: C 59.22%, H 4.97%, N 10.90%, O 24.91%
Literature References: Dihydropyridine calcium channel blocker. Prepn: Y. Sato,
BE 879263;
idem, US 4338322 (1980, 1982 both to Fujisawa). Structural studies: A. Miyamae
et al., Chem. Pharm. Bull. 34, 3071 (1986). Determn in plasma and urine: Y. Tokuma
et al., J. Chromatogr. 415, 156 (1987). Preliminary pharmacokinetics and resolution of enantiomers:
eidem, J. Pharm. Sci. 76, 310 (1987). Pharmacokinetics in rabbits: Y. Nezasa
et al., Kankyo Igaku Kenkyusho Nenpo 38, 200 (1987),
C.A. 107, 89273q (1987). Mode of action: P. A. Molyvdas, N. Sperelakis,
J. Cardiovasc. Pharmacol. 8, 449 (1986). Cardiovascular effects: G. J. Gross
et al., Gen. Pharmacol. 14, 677 (1983). Clinical evaluation in hypertension: K. Mizuno
et al., Res. Commun. Chem. Pathol. Pharmacol. 52, 3 (1986).
Properties: Yellow prisms from ethanol, mp 148-150°.
Melting point: mp 148-150°
Derivative Type: (+)-Form
Properties: [a]D20 +222.42° (c = 1 in methanol).
Optical Rotation: [a]D20 +222.42° (c = 1 in methanol)
Derivative Type: (-)-Form
Properties: [a]D20 -219.62° (c = 1 in methanol).
Optical Rotation: [a]D20 -219.62° (c = 1 in methanol)
Therap-Cat: Antihypertensive; antianginal.
Keywords: Antianginal; Antihypertensive; Dihydropyridine Derivatives; Calcium Channel Blocker; Dihydropyridine Derivatives.