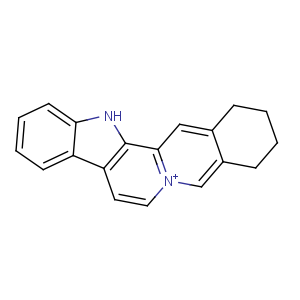
Title: Sempervirine
CAS Registry Number: 6882-99-1
CAS Name: 2,3,4,13-Tetrahydro-1
H-benz[
g]indolo[2,3-
a]quinolizin-6-ium
Synonyms: 3,4,5,6,14,15,20,21-octadehydroyohimbanium; sempervirene
Molecular Formula: C19H16N2
Molecular Weight: 272.34
Percent Composition: C 83.79%, H 5.92%, N 10.29%
Literature References: Found in rhizome and roots of Carolina jessamine (yellow jessamine)
Gelsemium sempervirens (L.) Ait. f.,
Loganiaceae: Stevenson, Sayre,
J. Am. Pharm. Assoc. 4, 60 (1915);
8, 708 (1919); Hasenfratz,
Bull. Soc. Chim. Fr. [4]
53, 1084 (1933); Forsyth
et al., J. Chem. Soc. 1945, 579; Prelog,
Helv. Chim. Acta 31, 588 (1948). Believed to exist primarily as inner salt depicted below: Woodward, Witkop,
J. Am. Chem. Soc. 71, 379 (1949). Synthesis: Woodward, McLamore,
ibid. 379; Swan,
J. Chem. Soc. 1958, 2039; Ban, Seo,
Tetrahedron 16, 11 (1961); Potts, Mattingly,
J. Org. Chem. 33, 3985 (1968).
Derivative Type: Monohydrate
Properties: Yellow needles from chloroform, mp 228°. Brownish-yellow leaflets from dil ethanol, mp 258°. uv max (ethanol): 243, 249, 297, 345, 387 nm (log e 4.58, 4.57, 4.20, 4.26, 4.24). Dipole moment: 8.5 (dioxane); 7.5 (benzene). Soluble in alcohol, chloroform, pyridine. Slightly sol in acetone. Practically insol in ether, benzene.
Melting point: mp 228°; mp 258°
Absorption maximum: uv max (ethanol): 243, 249, 297, 345, 387 nm (log e 4.58, 4.57, 4.20, 4.26, 4.24)
Derivative Type: Methochloride
Molecular Formula: C20H19ClN2
Molecular Weight: 322.83
Percent Composition: C 74.41%, H 5.93%, Cl 10.98%, N 8.68%
Properties: Minute yellow needles from ethanol, mp 330-332°. Soluble in water giving yellow solns with purple fluorescence. uv max: 241, 292, 330, 395 nm (log e 4.56, 4.20, 4.28, 4.22).
Melting point: mp 330-332°
Absorption maximum: uv max: 241, 292, 330, 395 nm (log e 4.56, 4.20, 4.28, 4.22)