References of Oxayohimban-16-carboxylicacid, 16,17-didehydro-19-methyl-, methyl ester, (19a)-
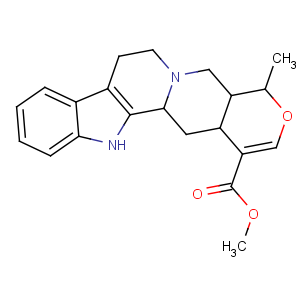
Title: Raubasine
CAS Registry Number: 483-04-5
CAS Name: (19a)-16,17-Didehydro-19-methyloxayohimban-16-carboxylic acid methyl ester
Synonyms: d-yohimbine; pytetrahydroserpentine; tetrahydroserpentine; ajmalicine
Trademarks: Circolene (Inverni); Hydrosarpan (Eutherapie); Isoarteril (Isola-Ibi); Lamuran (Boehringer, Mann.)
Molecular Formula: C21H24N2O3
Molecular Weight: 352.43
Percent Composition: C 71.57%, H 6.86%, N 7.95%, O 13.62%
Literature References: a1-Adrenergic blocker isolated from bark of
Corynanthe johimbe K. Schum.,
Rubiaceae: H. Heinemann,
Ber. 67, 15 (1934); from roots of
Rauwolfia serpentina (L.) Benth.,
Apocynaceae: S. Siddiqui, R. H. Siddiqui,
J. Indian Chem. Soc. 8, 667 (1931); A. H. Popelak
et al., Naturwissenschaften 40, 625 (1953); A. Hofmann,
Helv. Chim. Acta 37, 849 (1954); M. W. Klohs
et al., J. Am. Chem. Soc. 76, 1332 (1954). Review of early literature: R. E. Woodson
et al., Rauwolfia: Botany, Pharmacognosy, Chemistry and Pharmacology (Little, Brown and Co., Boston, 1957) 147 pp. Structure: Goutarel, Le Hir,
Bull. Soc. Chim. Fr. 18, 909 (1951). Stereochemistry: Wenkert
et al., J. Am. Chem. Soc. 83, 5037 (1961); Shamma, Richey,
ibid. 85, 2507 (1963). Total synthesis of
dl-form: van Tamelen, Placeway,
ibid. 83, 2594 (1961); van Tamelen
et al., ibid. 91, 7359 (1969); J. Gutzwiller
et al., Helv. Chim. Acta 64, 1663 (1981); T. Kametani
et al., J. Chem. Soc. Perkin Trans. 1 1981, 3168. Biosynthesis: N. Nagakura
et al., ibid. 1979, 2308; M. Rueffer
et al., Chem. Commun. 1979, 1016. Pharmacokinetics: A. Marzo
et al., Farmaco Ed. Prat. 36, 173 (1981). Clinical evaluation of platelet anti-aggregant activity: J. Neuman
et al., Arzneim.-Forsch. 36, 1394 (1986). Evaluation of combination with almitrine,
q.v., in cerebral ischemia in rats: M. G. Borzeix, J. Cahn,
ibid. 37, 491 (1987).
Properties: Prisms from methanol, dec 257°. [a]D20 -60° (c = 0.5 in chloroform); [a]D20 -45° (c = 0.5 in pyridine); [a]D20 -39° (c = 0.25 in methanol). uv max (methanol): 227, 292 nm (log e 4.61, 3.79).
Optical Rotation: [a]D20 -60° (c = 0.5 in chloroform); [a]D20 -45° (c = 0.5 in pyridine); [a]D20 -39° (c = 0.25 in methanol)
Absorption maximum: uv max (methanol): 227, 292 nm (log e 4.61, 3.79)
Derivative Type: Hydrochloride
Molecular Formula: C21H24N2O3.HCl
Molecular Weight: 388.89
Percent Composition: C 64.86%, H 6.48%, N 7.20%, O 12.34%, Cl 9.12%
Properties: Leaflets from ethanol, mp 290° (dec). [a]D20 -17° (c = 0.5 in methanol). Sparingly sol in water or dil HCl.
Melting point: mp 290° (dec)
Optical Rotation: [a]D20 -17° (c = 0.5 in methanol)
Derivative Type: Hydrobromide
Molecular Formula: C21H24N2O3.HBr
Molecular Weight: 433.34
Percent Composition: C 58.20%, H 5.81%, N 6.46%, O 11.08%, Br 18.44%
Properties: Diamond-shaped platelets from methanol, mp 295-296°.
Melting point: mp 295-296°
Therap-Cat: Antihypertensive, anti-ischemic (cerebral and peripheral).
Keywords: Antihypertensive.