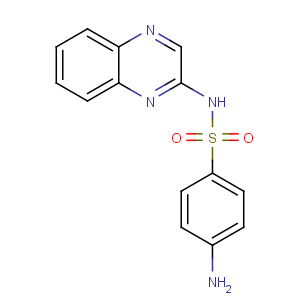
Title: Sulfaquinoxaline
CAS Registry Number: 59-40-5
CAS Name: 4-Amino-
N-2-quinoxalinylbenzenesulfonamide
Synonyms: N1-(2-quinoxalinyl)sulfanilamide; 2-sulfanilamidoquinoxaline;
N1-(2-quinoxalyl)sulfanilamide; sulfabenzpyrazine
Manufacturers' Codes: Compd 3-120
Trademarks: S.Q. (Merck & Co.); Sulquin (Salsbury)
Molecular Formula: C14H12N4O2S
Molecular Weight: 300.34
Percent Composition: C 55.99%, H 4.03%, N 18.65%, O 10.65%, S 10.68%
Literature References: Prepd by treating 2-aminoquinoxaline with acetylsulfanilyl chloride in the presence of pyridine and hydrolyzing the resulting acetyl deriv: Weijlard
et al., J. Am. Chem. Soc. 66, 1957 (1944); Weijlard, Tishler,
US 2404199 (1946 to Merck & Co.). Anticoccidial spectrum: G. F. Mathis
et al., Poult. Sci. 63, 1149 (1984). Evaluation of antibacterial and anticoccidial efficacy of mixture with trimethoprim: G. White, R. B. Williams,
Vet. Rec. 113, 608 (1983); D. W. T. Piercy
et al., ibid. 114, 60 (1984). Drug residue study in poultry muscle and liver: M. Patthy,
J. Chromatogr. 275, 115 (1983). HPLC determn in rabbit plasma and urine: J. G. Eppel, J. J. Thiessen,
J. Pharm. Sci. 73, 1635 (1984).
Properties: Minute crystals, mp 247-248°. uv max (pH 6.6 in H2O): 252, 360 nm (E1%1cm 1110, 275). Solubility in water at pH 7: 0.75 mg/100 ml; in 95% alcohol: 73 mg/100 ml; in acetone: 430 mg/100 ml. Sol in aq Na2CO3 and NaOH solns.
Melting point: mp 247-248°
Absorption maximum: uv max (pH 6.6 in H2O): 252, 360 nm (E1%1cm 1110, 275)
Derivative Type: Sodium salt
Trademarks: Aviochina (Agrimont-Vetem)
Molecular Formula: C14H11N4NaO2S
Molecular Weight: 322.32
Percent Composition: C 52.17%, H 3.44%, N 17.38%, Na 7.13%, O 9.93%, S 9.95%
Properties: Very sol in water; pH of 1% soln ~10. The amorphous salt is deliquescent and absorbs CO2 which liberates the practically insol sulfaquinoxaline.
Therap-Cat-Vet: Coccidiostat.