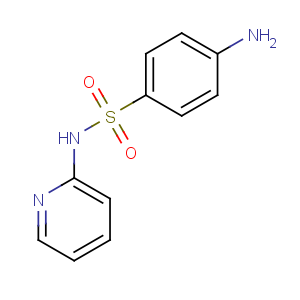
Title: Sulfapyridine
CAS Registry Number: 144-83-2
CAS Name: 4-Amino-
N-2-pyridinylbenzenesulfonamide
Synonyms: dagenan;
N1-2-pyridylsulfanilamide; 2-sulfanilamidopyridine; 2-(
p-aminobenzenesulfonamido)pyridine
Manufacturers' Codes: M & B 693
Molecular Formula: C11H11N3O2S
Molecular Weight: 249.29
Percent Composition: C 53.00%, H 4.45%, N 16.86%, O 12.84%, S 12.86%
Literature References: Prepn: A. J. Ewins, M. A. Phillips,
GB 512145 (1939);
eidem, US 2275354 (1942); R. Winterbottom,
J. Am. Chem. Soc. 62, 160 (1940). Metabolism: R. Wien, J. Hampton,
J. Pharmacol. Exp. Ther. 84, 211 (1945). Toxicology: R. Wien
et al., ibid. 203. GC-MS determn in edible animal tissues: A. E. Mooser, H. Koch,
J. AOAC Int. 76, 976 (1993). Efficacy and proposed mechanism of action in dermatological disease: O. J. Stone,
Med. Hypotheses 31, 99 (1990). Clinical evaluation in ocular cicatricial pemphigoid: M. J. Elder
et al., Br. J. Ophthalmol. 80, 549 (1996).
Properties: Crystals from alc, mp 190-191°. One gram dissolves in ~3500 ml water, 440 ml alcohol, 65 ml acetone. Freely sol in dil mineral acids and in aq solns of KOH and NaOH. More sol in warm sugar solns than in water alone. The aq soln is neutral. LD50 orally in mice: 7.5 mg/g (Wien).
Melting point: mp 190-191°
Toxicity data: LD50 orally in mice: 7.5 mg/g (Wien)
Derivative Type: Sodium salt monohydrate
CAS Registry Number: 127-57-1
Synonyms: Soluble sulfapyridine
Molecular Formula: C11H10N3NaO2S.H2O
Molecular Weight: 289.29
Percent Composition: C 45.67%, H 4.18%, N 14.53%, Na 7.95%, O 16.59%, S 11.08%
Properties: On prolonged exposure to humid air it absorbs CO2, liberates sulfapyridine, becomes incompletely sol in water. One gram dissolves in ~1.5 ml water, 10 ml alcohol. pH of 5% aq soln: 11.4. LD50 orally in mice: 2.7 mg/g (Wien).
Toxicity data: LD50 orally in mice: 2.7 mg/g (Wien)
Therap-Cat: In treatment of dermatitis herpetiformis; antibacterial.
Therap-Cat-Vet: Antibacterial.
Keywords: Antibacterial (Synthetic); Sulfonamides; Dermatitis Herpetiformis Suppressant.