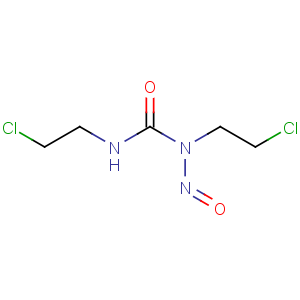
Title: Carmustine
CAS Registry Number: 154-93-8
CAS Name: N,N¢-Bis(2-chloroethyl)-
N-nitrosourea
Synonyms: BCNU
Manufacturers' Codes: NSC-409962
Trademarks: Becenun (BMS); Bicnu (BMS); Carmubris (BMS)
Molecular Formula: C5H9Cl2N3O2
Molecular Weight: 214.05
Percent Composition: C 28.06%, H 4.24%, Cl 33.13%, N 19.63%, O 14.95%
Literature References: Chloroethylnitrosourea derivative with antitumor activity. Similar to chlorozotocin, lomustine, nimustine, ranimustine,
q.q.v. Synthesis: Johnston
et al., J. Med. Chem. 6, 669 (1963). Properties: Loo
et al., J. Pharm. Sci. 55, 492 (1966). Decompn studies as related to antileukemic activity: Montgomery
et al., J. Med. Chem. 10, 668 (1967). Antifungal action: Hunt, Pittilo,
Antimicrob. Agents Chemother. 1965, 710. Toxicology studies: Thompson, Larson,
Toxicol. Appl. Pharmacol. 21, 405 (1972). Review of pulmonary toxicity: A. C. Smith,
Pharmacol. Ther. 41, 443-460 (1989).
Properties: Light yellow powder that melts to an oily liquid; mp 30-32°. Both powder and liquid are stable. Dec rapidly in acid and in soln above pH 7. Most stable in petroleum ether or aqueous soln at pH 4. Non-ionized at pH 7 with consequent high lipid solubility. Sol in water up to 4 mg/ml and in 50% ethanol up to 150 mg/ml: DeVita
et al., Cancer Res. 25, 1876 (1965). LD50 in mice (mg/kg): 19-25 orally, 26 i.p., 24 s.c.; in rats (mg/kg): 30-34 orally (Thompson, Larson).
Melting point: mp 30-32°
Toxicity data: LD50 in mice (mg/kg): 19-25 orally, 26 i.p., 24 s.c.; in rats (mg/kg): 30-34 orally (Thompson, Larson)
CAUTION: This substance is reasonably anticipated to be a human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-53.
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Alkylating Agents; Nitrosoureas.