References of 6,14-Ethenomorphinan-7-methanol,17-(cyclopropylmethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-a,a-dimethyl-, (5a,7a)-
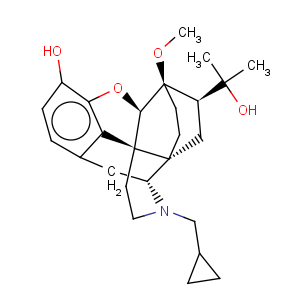
Title: Diprenorphine
CAS Registry Number: 14357-78-9
CAS Name: (5a,7a)-17-(Cyclopropylmethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-a,a-dimethyl-6,14-ethenomorphinan-7-methanol
Synonyms: 21-cyclopropyl-6,7,8,14-tetrahydro-7a-(1-hydroxy-1-methylethyl)-6,14-
endo-ethanooripavine;
N-(cyclopropylmethyl)-19-methylnororvinol
Manufacturers' Codes: M-5050; RX-5050M
Molecular Formula: C26H35NO4
Molecular Weight: 425.56
Percent Composition: C 73.38%, H 8.29%, N 3.29%, O 15.04%
Literature References: Closely related to cyprenorphine,
q.v. Prepn: K. W. Bentley, D. G. Hardy,
J. Am. Chem. Soc. 89, 3281 (1967). Activity in rats: G. F. Blane,
J. Pharm. Pharmacol. 19, 367 (1967); G. F. Blane, D. Dugdall,
ibid. 20, 547 (1968). Use as etorphine antagonist in dogs: M. Grange
et al., Rev. Med. Vet. 124, 899 (1973); in large animals: B. T. Alford
et al., J. Am. Vet. Med. Assoc. 164, 702 (1974). Binding to opiate receptors: C. B. Pert
et al., Life Sci. 16, 1849 (1975); J. Pearson
et al., ibid. 26, 1047 (1980); to m opiate receptors: J. J. Frost
et al., ibid. 38, 1597 (1986). Treatment of experimental stroke in cats: D. S. Baskin
et al., Neuropeptides 5, 307 (1985);
eidem, J. Neurosurg. 64, 99 (1986). Determn by HPLC: I. Jane, A. McKinnon,
J. Chromatogr. 323, 191 (1985). Toxicity data: N. S. Duggett
et al., Toxicol. Appl. Pharmacol. 31, 141 (1977).
Properties: Crystals from methanol, mp 185°.
Melting point: mp 185°
Derivative Type: Hydrochloride
Trademarks: Revivon (Reckitt & Colman)
Molecular Formula: C26H35NO4.HCl
Molecular Weight: 462.02
Percent Composition: C 67.59%, H 7.85%, N 3.03%, O 13.85%, Cl 7.67%
Properties: LD50 s.c. in mice: 316.0 ± 20 mg/kg (Duggett).
Toxicity data: LD50 s.c. in mice: 316.0 ± 20 mg/kg (Duggett)
Therap-Cat-Vet: Narcotic antagonist.