Levodopa
-
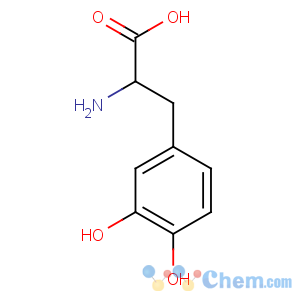
Molecular Structure
Detailed Description
Levodopa
Alias: ;HYDROXYTYROSINEL-DOPA, L-3-HYDROXYTYROSINE
Product Details:
Place of Origin: China
Certification: ISO9001
Brand:YuanCheng
Payment & Shipping Terms:
Minimum Order Quantity:1kg
Supply Ability:500kg/month
Delivery Time:within 24 hours after payment
Payment Terms:Western Union, MoneyGram, T/T
Package:25 kg/drum
Appearance:White crystalline Powder
Product Description:
CAS: 59-92-7
EINECS: 200-445-2
M.F.: C9H11NO4
M.W. : 197.19
Purity:99%
M.P.: 276-278 °C
M.S.:
L-DOPAis a chemical that is made and used as part of the normal biology of humans, some animals and plants. Some animals and humans make it via biosynthesis from the amino acid L-tyrosine. L-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) collectively known as catecholamines. L-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Parcopa, Atamet, Stalevo, Madopar, and Prolopa. As a drug it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.
L-DOPA crosses the protective blood-brain barrier, whereas dopamine itself cannot. Thus, L-DOPA is used to increase dopamine concentrations in the treatment of Parkinson's disease and dopamine-responsive dystonia. This treatment was made practical and proven clinically by George Cotzias and his coworkers, for which they won the 1969 Lasker Prize.[1][2] Once L-DOPA has entered the central nervous system, it is converted into dopamine by the enzyme aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC). Pyridoxal phosphate (vitamin B6) is a required cofactor in this reaction, and may occasionally be administered along with L-DOPA, usually in the form of pyridoxine.
Besides the central nervous system, L-DOPA is also converted into dopamine from within the peripheral nervous system. The resulting hyperdopaminergia causes many of the adverse side effects seen with sole L-DOPA administration. To bypass these effects, it is standard clinical practice to co-administer (with L-DOPA) a peripheral DOPA decarboxylase inhibitor (DDCI) such as carbidopa (medicines combining L-DOPA and carbidopa are branded as Lodosyn, Sinemet, Parcopa, Atamet, Stalevo) or with a benserazide (combination medicines are branded Madopar, Prolopa), to prevent the peripheral synthesis of dopamine from L-DOPA. Co-administration of pyridoxine without a DDCI accelerates the peripheral decarboxylation of L-DOPA to such an extent that it negates the effects of L-DOPA administration, a phenomenon that historically caused great confusion.
Usage:
Parkinsonism effective drug treatment, mainly for Parkinson's disease, etc.
Parkinsonism: mild to moderate illness who is better, or elderly patients with severe efficiency is poor. 2 hepatic coma: can awake patient, symptoms improved. Hepatic coma may be associated with abnormalities of central neurotransmitter dopamine, after use, can be improved and effective central function. 3 Some people think that levodopa may improve brain ammonia tolerance.
M.P.: 276-278 °C
ychz01(at)yccreate dot com

- Levodopa




