2-Deoxy-D-glucose
-
- Product Name2-Deoxy-D-glucose
- CAS No.154-17-6
- Purity99%
- Min Quantity
- Price0~100

 View Contact Detail
View Contact Detail
-
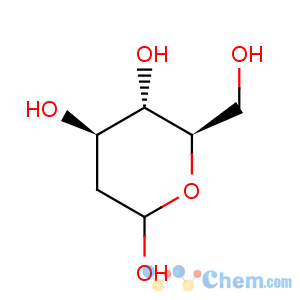 Molecular Structure
Molecular Structure

- 2-Deoxy-D-glucose
Detailed Description
2-Deoxy-D-glucoseproduct Name: 2-Deoxy-D-glucose
Synonyms: 2-deoxy-D-glucose, mixture of anomers; 2-deoxy-D-glucose sigmaultra; 2-deoxy-D-glucose grade iii; 2-deoxy-D-glucose grade ii; 2-Deoxy-D-Arabino; Deoxy-d-glucose; 2-deoxyhexopyranose; 2-deoxy-alpha-D-arabino-hexopyranose; 2-deoxy-D-arabino-hexose; 2-deoxy-D-arabino-hexopyranose; 2-deoxy-beta-D-arabino-hexopyranose
Molecular Formula: C6H12O5
Molecular Weight: 164.1565
CAS Registry Number: 154-17-6
EINECS: 205-823-0
Packing:25kg/drum
Assay:99%
Description
--------------------------------------------------------
2-Deoxy-D-glucose is a glucose molecule which has the 2-hydroxyl group replaced by hydrogen, so that it cannot undergo further glycolysis. As such, it acts to competitively inhibit the production of glucose-6-PO4 from glucose at the phosphoglucoisomerase level.[2] In most cells, glucose hexokinase phosphorylates 2-deoxyglucose, trapping the product 2-deoxyglucose-6-phosphate intracellularly (with exception of liver and kidney)[citation needed]; thus, labeled forms of 2-deoxyglucose serve as a good marker for tissue glucose uptake and hexokinase activity. Many cancers have elevated glucose uptake and hexokinase levels. 2-Deoxyglucose labeled with tritium or carbon-14 has been a popular ligand for laboratory research in animal models, where distribution is assessed by tissue-slicing followed by autoradiography, sometimes in tandem with either conventional or electron microscopy.
2-DG is uptaken by the glucose transporters of the cell. Therefore, cells with higher glucose uptake, for example tumor cells, have also a higher uptake of 2-DG. Since 2-DG hampers cell growth, its use as a tumor therapeutic has been suggested, and in fact, 2-DG is in clinical trials [3] A recent clinical trial showed 2-DG can be tolerated at a dose of 63 mg/kg/day, however the observed cardiac side-effects (prolongation of the Q-T interval) at this dose and the fact that a majority of patients' (66%) cancer progressed casts doubt on the feasibility of this reagent for further clinical use.[4] However, it is not completely clear how 2-DG inhibits cell growth. The fact that glycolysis is inhibited by 2-DG, seems not to be sufficient to explain why 2-DG treated cells stop growing [5]
Clinicians have noted that 2-DG is metabolised in the pentose phosphate pathway in red blood cells at least, although the significance of this for other cell types and for cancer treatment in general is unclear.[6]
Work on the ketogenic diet as a treatment for epilepsy have investigated the role of glycolysis in the disease. 2-Deoxyglucose has been proposed by Garriga-Canut et al. as a mimic for the ketogenic diet, and shows great promise as a new anti-epileptic drug.[7] The authors suggest that 2-DG works, in part, by increasing the expression of Brain-derived neurotrophic factor (BDNF), Nerve growth factor (NGF), Arc (protein) (ARC), and Basic fibroblast growth factor (FGF2).[8] Such uses are complicated by the fact that 2-deoxyglucose does have some toxicity.
Chemical Uses
-------------------------------------------------------
2-DG has been used as a targeted optical imaging agent for fluorescent in vivo imaging.[9][10] In clinical medical imaging (PET scanning), fluorodeoxyglucose is used, where one of the 2-hydrogens of 2-deoxy-D-glucose is replaced with the positron-emitting isotope fluorine-18, which emits paired gamma rays, allowing distribution of the tracer to be imaged by external gamma camera(s). This is increasingly done in tandem with a CT function which is part of the same PET/CT machine, to allow better localization of small-volume tissue glucose-uptake differences.