Chlormadinone acetate
-
- Product NameChlormadinone acetate
- CAS No.302-22-7
- Purity99%
- Min QuantityKilograms
- Price0~100

 View Contact Detail
View Contact Detail
-
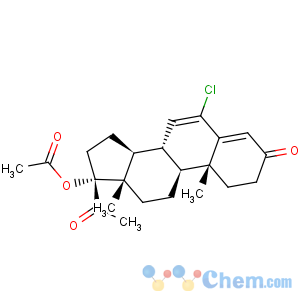 Molecular Structure
Molecular Structure

- Chlormadinone acetate
Detailed Description
Quick Details:--------------------------------------------
Chlormadinone acetate
Synonyms: 17-(Acetyloxy)-6-chloropregna-4,6-diene-3,20-dione
CAS: 302-22-7
MF: C23H29ClO4
MW: 404.93
Standard: CP2000, JP13
Appearance: white crystalline powder
Assay: 99%
Packing: Foil bag
Usage: Orally active progesteron with antiandrogenic activity; has been used in combinations as an oral contraceptive. Progestogen; antineoplastic (hormonal)
Description
--------------------------------------------
Chlormadinone acetate (INN, USAN, BAN, JAN; sold under brand names including Clordion, Gestafortin, Lormin, Non-Ovlon, Normenon, Verton, and many others), sometimes abbreviated as CMA, and also known as 17α-acetoxy-6-chloro-6-dehydroprogesterone, is a steroidal progestin with additional antiandrogen and antigonadotropic (and thus also antiestrogenic) effects. It is used clinically as a hormonal contraceptive, and in part due to its lowering of estrogen levels, but also for improved effectiveness in contraception, chlormadinone is frequently combined with ethinyl estradiol for this purpose.It is the acetate ester of chlormadinone.
Uses
--------------------------------------------Chlormadinone acetate has not been used in the United States since 1970, when the only product (an oral contraceptive) was removed from the market. Its use in the United Kingdom was suspended in the same year. Before suspension, chlormadinone acetate was used in oral contraceptives either together with mestranol as a "sequential" contraceptive or as a "progestogen only" oral contraceptive. Chlormadinone acetate has been used (frequently in combination with mestranol) for treatment of threatened abortion and dysmenorrhea.
Ethinylestradiol/chlormadinone acetate 0.03/2mg (EE/CMA) is a combined monophasic contraceptive pill with anti-androgenic properties. In a large, non-comparative, multicentre trial (< or =24 cycles of treatment per woman) and two (6- and 12-cycle) post-marketing surveillance studies, EE/CMA was effective in preventing pregnancy. EE/CMA was significantly more effective than EE/levonorgestrel 0.03/0.15 mg/day in treating women with mild-to-moderate papulopustular acne of the face and related disorders in a randomized, single-blind, multicentre trial. EE/CMA was well tolerated in clinical trials and the post-marketing surveillance studies. Adverse events were those commonly reported with oral contraceptives. As expected, the most common menstrual disturbances were breakthrough bleeding, spotting and amenorrhea.