Rapamycin
-
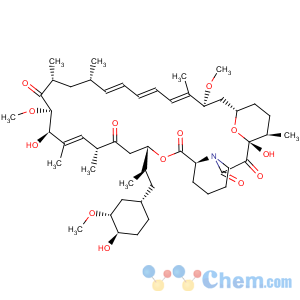
Molecular Structure
Detailed Description
Rapamycin
Synonyms:23,27-epoxy-3h-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine;Nsc-226080;Rapa;Rapamune;Rapamycin;Rapamycin, streptomyces hygroscopicus;Rpm
Cas:53123-88-9
Mf:C51h79no13
Mw:914.18
Einecs:262-640-9
Product categories:Active pharmaceutical ingredients;Immunosuppressant;Pharmaceuticals;Cytokine signaling;Chiral reagents;Heterocycles;Inhibitor;Vivactil;Antibiotic
Chemical properties:White to off-white solid
Assay:99%
Usage:A triene macrolide antibiotic isolated from streptomyces hygroscopicus.Name derived from the native word for easter island, rapa'nui.Used as an immunosuppressant;Antirestenotic.Rapamycin is a triene macrolide discovered in 1995 as a metabolite of streptomyces hygroscopicus found in a soil obtained on rapi nui (easter island).Rapamycin displayed potent and selective antifungal activity, notably against candida albicans.Interest in the metabolite waned until the structural relationship to the potent immunosuppressant fujimycin (antibiotic fk506) was recognised in the mid-1980s.This recognition led to the re-discovery of rapamycin as a highly selective antitumour and immunosuppressant.Rapamycin inhibits the activity of the protein, mtor (mammalian target of rapamycin) which functions in a signalling pathway to promote tumour growth.Rapamycin binds to a receptor protein (fkbp12).The rapamycin/fkb12 complex then binds to mtor and prevents interaction of mtor with target proteins in this signalling pathway.
Packing:Foil bag or as required
Content: >99%
Appearance: A white,Crystalline Powder
Formula: C51H79NO13
Molecular Weight 914.18
Moisture: <1mg/mL
Loss on Drying: ≤0.2%
Residue on ignition: ≤0.1%
Stability: at -20℃2 years
Heavy Metals: ≤10PPM
Acidity or Alkalinity: Conform
Chloride: Conform
Readily Carbonizable Substances: Conform
Ordinary Impurities: Conform
Item Specifications Results
Description off-white to yellow crystalline powder Complies
Identification The IR spectrum of Potassium Bromide preparation of the sample exhibits maxima at the same wavelengths as that of a similar preparation of in-house reference standard Complies
The UV spectrum of 95% Ethanol preparation of the sample exhibits maxima at 267nm, 277nm, 288nm that of similar preparation of in-house reference standard. Complies
The retention times of the peaks for the Trans- and Cis-stereoisomers in the chromatogram of Assay preparation corresponds to that in the chromatogram of the standard preparation, as obtained in the Assay Complies
Loss on drying ≤0.5% 0.16%
Heavy metal ≤0.002% Complies
Total impurity ≤2.0% 0.76%
Single impurity ≤1.0% 0.67%
Cis-stereoisomer of Sirolimus ≤5.0% 2.4%
Assay ≥98.0% 99.2%

- Rapamycin




