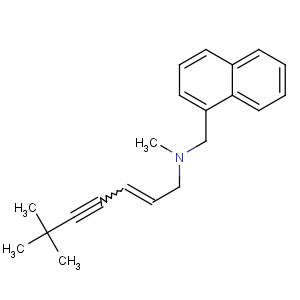
References of (E)-N,6,6-trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine
Title: Terbinafine
CAS Registry Number: 91161-71-6
CAS Name: N-[(2
E)-6,6-Dimethyl-2-hepten-4-ynyl]-
N-methyl-1-naphthalenemethanamine
Synonyms: trans-N-methyl-
N-(1-naphthylmethyl)-6,6-dimethylhept-2-en-4-ynyl-1-amine
Molecular Formula: C21H25N
Molecular Weight: 291.43
Percent Composition: C 86.55%, H 8.65%, N 4.81%
Literature References: Orally active, antimycotic allylamine related to naftifine,
q.v. Specific inhibitor of squalene epoxidase, a key enzyme in fungal ergosterol biosynthesis. Prepn: A. Stütz,
EP 24587;
idem, US 4755534 (1981, 1988 both to Sandoz); A. Stütz, G. Petranyi,
J. Med. Chem. 27, 1539 (1984). Mode of action: G. Petranyi
et al., Science 224, 1239 (1984); N. S. Ryder,
Antimicrob. Agents Chemother. 27, 252 (1985).
In vitro antifungal activity: S. Shadomy
et al., Sabouraudia 23, 125 (1985). Toxicity data: U. Ganzinger
et al., Proc. 13th Int. Congr. Chemother. 6, 116/52 (1983). Symposium on pharmacology and clinical trials:
J. Am. Acad. Dermatol. 23, Suppl., 775-812 (1990). Clinical trial as systemic treatment of toenail onychomycosis: E. G. V. Evans
et al., Br. Med. J. 318, 1031 (1999).
Derivative Type: Hydrochloride
CAS Registry Number: 78628-80-5
Manufacturers' Codes: SF-86-327
Trademarks: Lamisil (Novartis)
Molecular Formula: C21H25N.HCl
Molecular Weight: 327.89
Percent Composition: C 76.92%, H 7.99%, N 4.27%, Cl 10.81%
Properties: Crystals from 2-propanol + diethyl ether, mp 195-198° (change in crystal structure begins ~150°). Freely sol in methanol, methylene chloride; sol in ethanol; slightly sol in water. LD50 in mice, rats (mg/kg): 4000, 4000 orally; 393, 213 i.v. (Ganzinger).
Melting point: mp 195-198° (change in crystal structure begins ~150°)
Toxicity data: LD50 in mice, rats (mg/kg): 4000, 4000 orally; 393, 213 i.v. (Ganzinger)
Therap-Cat: Antifungal.
Keywords: Antifungal (Synthetic); Allylamines.