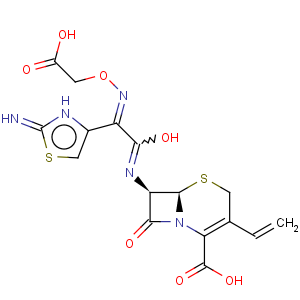
Title: Cefixime
CAS Registry Number: 79350-37-1
CAS Name: (6
R,7
R)-7-[[(2
Z)-(2-Amino-4-thiazolyl)[(carboxymethoxy)imino]acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Synonyms: 7-[2-(2-amino-4-thiazolyl)-2-(carboxymethoxyimino)acetamido]-3-vinyl-3-cephem-4-carboxylic acid
Manufacturers' Codes: FK-027; FR-17027; CL-284635
Trademarks: Cefixoral (Menarini); Cefspan (Fujisawa); Cephoral (Merck KGaA); Oroken (Bellon); Suprax (Wyeth); Unixime (Firma)
Molecular Formula: C16H15N5O7S2
Molecular Weight: 453.45
Percent Composition: C 42.38%, H 3.33%, N 15.44%, O 24.70%, S 14.14%
Literature References: Orally active, third generation cephalosporin antibiotic. Prepn: T. Takaya
et al., EP 30630;
eidem, US 4409214 (1981, 1983 both to Fujisawa); H. Yamanaka
et al., J. Antibiot. 38, 1738 (1985). Synthesis and activity of
(E)-isomer: K. Kawabata
et al., Chem. Pharm. Bull. 34, 3458 (1986). Mechanism of action: Y. Shigi
et al., J. Antibiot. 37, 790 (1984). Comparative antibacterial spectrum
in vitro and
in vivo: T. Kamimura
et al., Antimicrob. Agents Chemother. 25, 98 (1984).
In vitro activity and b-lactamase stability: H. C. Neu
et al., ibid. 26, 174 (1984). HPLC determn in human plasma and urine: Y. Tokuma
et al., J. Chromatogr. 311, 339 (1984). Pharmacokinetics in humans: D. R. P. Guay
et al., Antimicrob. Agents Chemother. 30, 485 (1986). Clinical trial in urinary tract infections: J. Levenstein
et al., S. Afr. Med. J. 70, 455 (1986); A. Irvani
et al., Am. J. Med. 85, Suppl. 3A, 17 (1988); in respiratory infections: R. Kiani
et al., ibid. 6.
Derivative Type: (Z)-Form disodium salt
Molecular Formula: C16H13N5Na2O7S2
Molecular Weight: 497.41
Percent Composition: C 38.63%, H 2.63%, N 14.08%, Na 9.24%, O 22.52%, S 12.89%
Properties: mp >250°.
Melting point: mp >250°
Derivative Type: (E)-Form trihydrate
Properties: Pale yellow solid, mp 218-225° (dec).
Melting point: mp 218-225° (dec)
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Antibiotics); ?Lactams; Cephalosporins.