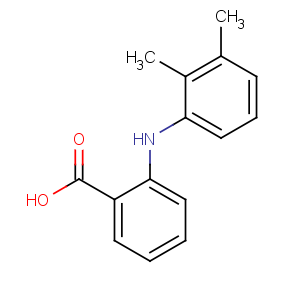
Title: Mefenamic Acid
CAS Registry Number: 61-68-7
CAS Name: 2-[(2,3-Dimethylphenyl)amino]benzoic acid
Synonyms: N-(2,3-xylyl)anthranilic acid
Manufacturers' Codes: CI-473; INF-3355
Trademarks: Lysalgo (SIT); Mefenacid (Streuli); Parkemed (Parke-Davis); Ponalar (Parke-Davis); Ponstan (Pfizer); Ponstel (Warner-Lambert); Ponstyl (Parke-Davis); Pontal (Parke-Davis)
Molecular Formula: C15H15NO2
Molecular Weight: 241.29
Percent Composition: C 74.67%, H 6.27%, N 5.80%, O 13.26%
Literature References: Prepn:
BE 605302; R. A. Scherrer,
US 3138636 (1961, 1964 both to Parke-Davis). Pharmacology: C. V. Winder
et al., J. Pharmacol. Exp. Ther. 138, 405 (1962); C. V. Winder
et al., Arthritis Rheum. 12, 472 (1969); and toxicology: Mokhort, Korkhova,
Farmakol. Toksikol. (Kiev) 1968, 85,
C.A. 71, 29080c (1969); U. Jahn, R. W. Adrian,
Arzneim.-Forsch. 19, 36 (1969). Crystal structure: J. F. McConnell, F. Z. Company,
Cryst. Struct. Commun. 5, 861 (1976). Clinical trial in primary dysmenorrhea: P. W. Budoff,
J. Am. Med. Assoc. 241, 2713 (1979). HPLC determn in human plasma: I. Niopas, K. Mamzoridi,
J. Chromatogr. B 656, 447 (1994).
Properties: Crystals, mp 230-231° (effervescence). pKa 4.2. uv max (0.l
N NaOH): 285, 340 nm. Soly in H2O, pH 7.1 (g/100 ml): 0.0041 (25°); 0.008 (37°). Soluble in solns of alkali hydroxides; sparingly sol in ether, chloroform; slightly sol in ethanol. LD50 orally in mice, rats: 630, 790 mg/kg (Jahn, Adrian).
Melting point: mp 230-231° (effervescence)
pKa: pKa 4.2
Absorption maximum: uv max (0.l
N NaOH): 285, 340 nm
Toxicity data: LD50 orally in mice, rats: 630, 790 mg/kg (Jahn, Adrian)
Derivative Type: Sodium salt
Molecular Formula: C15H14NNaO2
Molecular Weight: 263.27
Percent Composition: C 68.43%, H 5.36%, N 5.32%, Na 8.73%, O 12.15%
Properties: White powder. Soly in H2O: >5 g/100 ml. LD50 in mice (mg/kg): 600 orally; 150 i.p. (Mokhort, Korkhova).
Toxicity data: LD50 in mice (mg/kg): 600 orally; 150 i.p. (Mokhort, Korkhova)
Therap-Cat: Anti-inflammatory; analgesic.
Keywords: Anti-inflammatory (Nonsteroidal); Aminoarylcarboxylic Acid Derivatives.