Title: Pyrimethamine
CAS Registry Number: 58-14-0
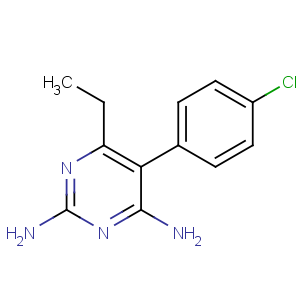
CAS Name: 5-(4-Chlorophenyl)-6-ethyl-2,4-pyrimidinediamine
Synonyms: 2,4-diamino-5-(
p-chlorophenyl)-6-ethylpyrimidine
Manufacturers' Codes: RP-4753
Trademarks: Daraprim (GSK); Malocide (Aventis)
Molecular Formula: C12H13ClN4
Molecular Weight: 248.71
Percent Composition: C 57.95%, H 5.27%, Cl 14.25%, N 22.53%
Literature References: Dihydrofolate reductase inhibitor; generally used in combination with other antimicrobial agents. Prepn: P. B. Russell, G. H. Hitchings,
J. Am. Chem. Soc. 73, 3763 (1951); G. H. Hitchings
et al., US 2576939 (1951 to Burroughs Wellcome); W. Logemann
et al., Ber. 87, 435 (1954); R. M. Jacob,
US 2680740 (1954 to Rh?ne-Poulenc). Review of antimicrobial activity and mechanism of action: Burchall in
Antibiotics vol. 3, J. W. Corcoran, F. E. Hahn, Eds. (Springer-Verlag, New York, 1975) pp 312-320. Comprehensive description: M. A. Loutfy, H. Y. Aboul-Enein,
Anal. Profiles Drug Subs. 12, 463-482 (1983). LC-MS determn in plasma: B. A. Sinnaeve
et al., J. Chromatogr. A 1076, 97 (2005). Clinical evaluations in toxoplasmosis in AIDS patients: C. Leport
et al., Am. J. Med. 84, 94 (1988); B. Dannemann
et al., Ann. Intern. Med. 116, 33 (1992). Review of clinical experience in malaria: H. M. McIntosh
et al., Ann. Trop. Med. Parasitol. 93, 265-270 (1998); C. V. Plowe
et al., Br. Med. J. 328, 545 (2004).
Properties: Crystals, mp 233-234° (capillary); mp 240-242° (copper block). Practically insol in water. Slightly sol in ethanol, (about 9 g/l), in dil HCl (about 5 g/l); sol in boiling ethanol (about 25 g/l). Very sparingly sol in propylene glycol and dimethylacetamide at 70°.
Melting point: mp 233-234° (capillary); mp 240-242° (copper block)
Derivative Type: Combination with sulfadoxine
CAS Registry Number: 37338-39-9
Trademarks: Fansidar (Roche)
Therap-Cat: Antiprotozoal (Toxoplasma); antimalarial.
Therap-Cat-Vet: Antiprotozoal (Toxoplasma).
Keywords: Antimalarial; Antiprotozoal (Toxoplasma).