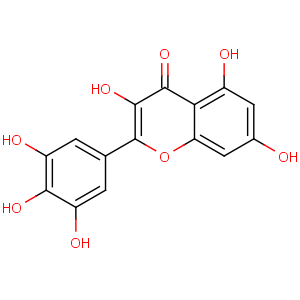
Title: Myricetin
CAS Registry Number: 529-44-2
CAS Name: 3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-4
H-1-benzopyran-4-one
Synonyms: 3,3¢,4¢,5,5¢,7-hexahydroxyflavone; cannabiscetin; delphidenolon 1575
Molecular Formula: C15H10O8
Molecular Weight: 318.24
Percent Composition: C 56.61%, H 3.17%, O 40.22%
Literature References: From the bark of
Myrica nagi Thunb.,
Myriaceae: Perkin, Hummel,
J. Chem. Soc. 69, 1287 (1896). Structure: Perkin,
ibid. 81, 203 (1902). Identity with cannabiscetin: Seshadri, Venkateswarlu,
Proc. Indian Acad. Sci. 23A, 296 (1946);
C.A. 40, 6447 (1946). Synthesis: Kalff, Robinson,
J. Chem. Soc. 127, 181 (1925); Rao
et al., J. Sci. Ind. Res. 8B, No. 6, 113 (1949). Occurrence in
Hamamelidaceae and
Anacardiaceae: Reznek, Egger,
Z. Naturforsch. 15b, 247 (1960). Metabolism: Smith, Griffiths,
Biochem. J. 118, 53p (1970).
Properties: Yellow needles from dil alc, mp 357°. uv max (ethanol): 375, 255 nm. Sparingly sol in boiling water; sol in alcohol. Practically insol in chloroform, acetic acid.
Melting point: mp 357°
Absorption maximum: uv max (ethanol): 375, 255 nm
Derivative Type: Hexaacetate
Molecular Formula: C27H22O14
Molecular Weight: 570.46
Percent Composition: C 56.85%, H 3.89%, O 39.27%
Properties: Crystals, mp 213°.
Melting point: mp 213°
Derivative Type: Hexaethyl ether
Molecular Formula: C27H34O8
Molecular Weight: 486.55
Percent Composition: C 66.65%, H 7.04%, O 26.31%
Properties: Needles from alcohol, mp 149-151°.
Melting point: mp 149-151°
Derivative Type: 3-Rhamnoside
Synonyms: Myricitrin
Molecular Formula: C21H20O12
Molecular Weight: 464.38
Percent Composition: C 54.31%, H 4.34%, O 41.34%
Properties: Structure: Hattori, Hayashi,
Acta Phytochim. 5, 213 (1931),
C.A. 26, 9908 (1932). Pale yellow leaflets from water, mp 199-200°. uv max (alc): 262, 352 nm. Sparingly sol in water, abs alcohol.
Melting point: mp 199-200°
Absorption maximum: uv max (alc): 262, 352 nm