References of Yohimban-16-carboxylicacid, 17-hydroxy-, methyl ester, (16a,17a,20a)-
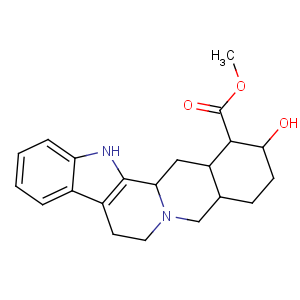
Title: allo-Yohimbine
CAS Registry Number: 522-94-1
CAS Name: 17-Hydroxyyohimban-16-carboxylic acid methyl ester
Synonyms: alloyohimbine; dihydroyohimbine
Molecular Formula: C21H26N2O3
Molecular Weight: 354.44
Percent Composition: C 71.16%, H 7.39%, N 7.90%, O 13.54%
Literature References: From bark of
Corynanthe johimbe K. Schum.,
Rubiaceae: Warnat,
Ber. 59, 2388 (1926); Hahn, Brandenberg,
ibid. 60, 699 (1927). Identity with dihydroyohimbine: Heinemann,
ibid. 67, 15 (1934). Structure and stereochemistry: LeHir
et al., Bull. Soc. Chim. Fr. 1953, 1027; Janot
et al., ibid. 1961, 637. Revised structure and total synthesis: T?ke
et al., J. Org. Chem. 38, 2496 (1973). Total synthesis: E. Wenkert
et al., J. Am. Chem. Soc. 101, 5370 (1979).
Review: A. Chatterjee
et al., "Rauwolfia Alkaloids" in Zechmeister,
Progress in the Chemistry of Organic Natural Products vol. XIII (Springer Verlag, Vienna, 1956) pp 354-356.
Properties: Trihydrate, needles from 50% alcohol, mp 98-99°. After drying at 100° and 0.01 mm Hg for 24 hrs, the anhydrous form melts at 135-140°. [a]D19 +84° (c = 0.40 in pyridine). uv max (methanol): 225, 280, 290 nm (log e 4.52, 3.91, 3.74). Sol in ethanol, methanol, pyridine, dil acids; slightly sol in benzene. Practically insol in water.
Melting point: mp 98-99°; anhydrous form melts at 135-140°
Optical Rotation: [a]D19 +84° (c = 0.40 in pyridine)
Absorption maximum: uv max (methanol): 225, 280, 290 nm (log e 4.52, 3.91, 3.74)