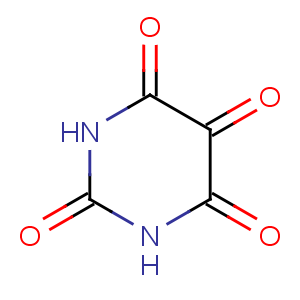
Title: Alloxan
CAS Registry Number: 50-71-5
CAS Name: 2,4,5,6(1
H,3
H)-pyrimidinetetrone
Synonyms: 2,4,5,6-tetraoxohexahydropyrimidine; mesoxalylurea; mesoxalylcarbamide
Molecular Formula: C4H2N2O4
Molecular Weight: 142.07
Percent Composition: C 33.82%, H 1.42%, N 19.72%, O 45.05%
Literature References: Found by Liebig in mucus excreted during dysentery. Prepn by direct oxidation of uric acid: G. Brugnatelli,
Ann. Chim. Phys. [2]
8, 201 (1818); J. Liebig, F. W?hler,
Ann. 26, 241 (1838); H. Biltz, M. Hehn,
ibid. 413, 60 (1917). Prepd from alloxantin: J. Liebig,
ibid. 147, 366 (1868); W. W. Hartman, O. E. Sheppard,
Org. Synth. coll. vol. III, 37 (1955).
See also A. V. Holmgren, W. W. Wenner,
ibid. coll. vol. IV, 23 (1963). Produces diabetes in animals by selective necrosis of pancreatic islet b-cells: J. S. Dunn, N. G. B. McLetchie,
Lancet 2, 384 (1943); W. B. Kennedy, F. D. W. Lukens,
Proc. Soc. Exp. Biol. Med. 57, 143 (1944). Mechanism of action study: L. Boquist,
Acta Pathol. Microbiol. Scand. Sect. A 88, 201 (1980).
In vitro antineoplastic activity: P. Grobon,
C.R. Seances Acad. Sci. Ser. D 280, 2413 (1975). Antibacterial and antifungal activity: J. D. Douros, A. F. Kerst,
JP Kokai 72 4900;
eidem, US 3728454 (1972, 1973 both to Gates Rubber Co.). Toxicological study in mice: B. A. Waisbren,
Proc. Soc. Exp. Biol. Med. 67, 154 (1948).
Properties: Anhydrous, orthorhombic crystals from anhydr acetone or glacial acetic acid or by sublimation in vacuo. Turns pink at 230° and dec at 256°. Acid to litmus. pK (25°) 6.63. Absorption spectrum: Hartley,
J. Chem. Soc. 87, 1802, 1808 (1905). Freely sol in water. Hot aqueous solns are yellow and become colorless on cooling. Aqueous solns, after being in contact with the human skin for some time, give it a red color and a disagreeable odor. Sol in acetone, alcohol, methanol, glacial acetic acid; slightly sol in chloroform, petr ether, toluene, ethyl acetate and acetic anhydride. Insol in ether.
pKa: pK (25°) 6.63
Derivative Type: Tetrahydrate
Properties: Large triclinic prisms or oblique monoclinic rhombs from water.
Derivative Type: Monohydrate
Properties: By heating the tetrahydrate at 100° or by exposing it to the air. Forms triclinic pinacoidal crystals.
Derivative Type: Compound with urea
Molecular Formula: CH4N2O.C4H2N2O4.H2O
Molecular Weight: 220.14
Percent Composition: C 27.28%, H 3.66%, N 25.45%, O 43.61%
Properties: Minute, yellow needles; red at 170°, decomp at 185-186°.
Use: In production of diabetes in experimental animals; in nutrition experiments; in organic syntheses.