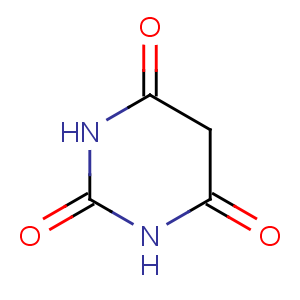
Title: Barbituric Acid
CAS Registry Number: 67-52-7
CAS Name: 2,4,6(1
H,3
H,5
H)-pyrimidinetrione
Synonyms: malonylurea; 2,4,6-trioxohexahydropyrimidine
Molecular Formula: C4H4N2O3
Molecular Weight: 128.09
Percent Composition: C 37.51%, H 3.15%, N 21.87%, O 37.47%
Literature References: Prepn from hydurilic acid + nitric acid: Baeyer,
Ann. 127, 199 (1863);
ibid. 130, 129 (1864). Structure: Mulder,
Ber. 6, 1233 (1873). Prepd from ethyl malonate and urea using sodium ethoxide as a condensing agent: Dickey, Gray,
Org. Synth. coll. vol. II, 60 (1943). Crystal structure: Bolton,
Nature 201, 987 (1964). Toxicity study: E. I. Goldenthal,
Toxicol. Appl. Pharmacol. 18, 185 (1971). Unsubstituted barbituric acid has no hypnotic properties.
Review: Carter,
J. Chem. Educ. 28, 524 (1951).
Derivative Type: Dihydrate
Properties: Rhombs from water. mp ~248° when anhydrous, with some decompn. Strong acid. K at 25° = 9.9 ′ 10-5. uv spectrum: Hartley,
J. Chem. Soc. 87, 1808 (1905). Difficultly sol in cold water; freely sol in hot water, in dil acids. Forms salts with metals. LD50 orally in male rats: >5000 mg/kg (Goldenthal).
Melting point: mp ~248° when anhydrous
Toxicity data: LD50 orally in male rats: >5000 mg/kg (Goldenthal)
Use: Manuf plastics, pharmaceuticals.