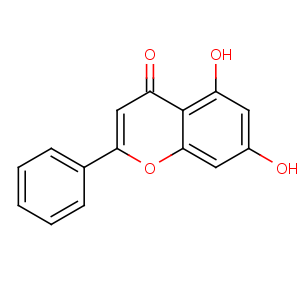
Title: Chrysin
CAS Registry Number: 480-40-0
CAS Name: 5,7-Dihydroxy-2-phenyl-4
H-1-benzopyran-4-one
Synonyms: 5,7-dihydroxyflavone; chrysidenon 1438
Molecular Formula: C15H10O4
Molecular Weight: 254.24
Percent Composition: C 70.86%, H 3.96%, O 25.17%
Literature References: From heartwood of
Pinus monticola Dougl.,
P. excelsa Wall., and
P. aristata Engelm.,
Pinaceae: Linstedt,
Acta Chem. Scand. 3, 1147, 1375 (1949);
4, 55 (1950); from bark of
Dolichandrone falcata Seem.,
Bisnomiaceae: Kincl,
Naturwissenschaften 42, 646 (1955). Synthesis: Seka, Prosche,
Monatsh. Chem. 69, 284 (1936); Hutchins, Wheeler,
J. Chem. Soc. 1939, 91; Teoule
et al., Bull. Soc. Chim. Fr. 1961, 546.
Properties: Light yellow prisms from methanol, mp 285°. uv max: 270, 329 nm (log e 4.40, 3.90). Practically insol in water; sol in alkali hydroxide solns; slightly sol in alcohol, chloroform, ether.
Melting point: mp 285°
Absorption maximum: uv max: 270, 329 nm (log e 4.40, 3.90)
Derivative Type: Diacetoxychrysin
Molecular Formula: C19H14O6
Molecular Weight: 338.31
Percent Composition: C 67.45%, H 4.17%, O 28.38%
Properties: Crystals from ethanol, mp 194-195°.
Melting point: mp 194-195°
Derivative Type: Methylchrysin
Synonyms: Tectochrysin
Molecular Formula: C16H12O4
Molecular Weight: 268.26
Percent Composition: C 71.64%, H 4.51%, O 23.86%
Literature References: It is present as such or in the form of a glucoside in buds of
Populus spp.,
Salicaceae. Use of tectochrysin as diuretic: Perrault,
US 3155579 (1964 to Laroche Navarron).
Properties: Yellow needles, mp 163°. Sol in alcohol, benzene, chloroform.
Melting point: mp 163°