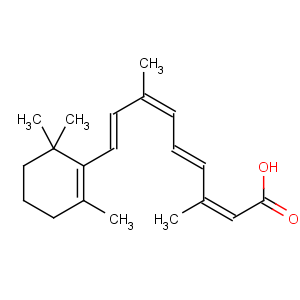
Title: Isotretinoin
CAS Registry Number: 4759-48-2
CAS Name: 13-
cis-Retinoic acid
Synonyms: 2-
cis-vitamin A acid; neovitamin A acid
Manufacturers' Codes: Ro-4-3780
Trademarks: Accutane (Roche); Isotrex (Stiefel); Oratane (Douglas); Roaccutane (Roche)
Molecular Formula: C20H28O2
Molecular Weight: 300.44
Percent Composition: C 79.95%, H 9.39%, O 10.65%
Literature References: Naturally occurring metabolite of vitamin A,
q.v.; inhibits sebum production. Prepn: C. D. Robeson
et al., J. Am. Chem. Soc. 77, 4111 (1955). Stereoselective process: R. Lucci,
EP 111325;
idem, US 4556518 (1984, 1985 both to Hoffmann-La Roche). Toxicology and teratogenicity study: J. J. Kamm,
J. Am. Acad. Dermatol. 6, 652 (1982). Identification as endogenous metabolite of all
-trans-retinoic acid: M. E. Cullum, M. H. Zile,
J. Biol. Chem. 260, 10590 (1985). HPLC determn in serum: G. Tang, R. M. Russell,
J. Lipid Res. 31, 175 (1990). Review of pharmacology and clinical efficacy in acne: A. R. Shalita
et al., Cutis 42, Suppl. 6A, 1-19 (1988). Symposium on clinical experience:
Dermatology 195, Suppl. 1, 1-37 (1997).
Properties: Reddish-orange plates from isopropyl alcohol, mp 174-175°. uv max: 354 nm (e 39800). LD50 (20 day) in mice, rats (mg/kg): 904, 901 i.p.; 3389, >4000 orally (Kamm).
Melting point: mp 174-175°
Absorption maximum: uv max: 354 nm (e 39800)
Toxicity data: LD50 (20 day) in mice, rats (mg/kg): 904, 901 i.p.; 3389, >4000 orally (Kamm)
Therap-Cat: Antiacne.
Keywords: Antiacne.