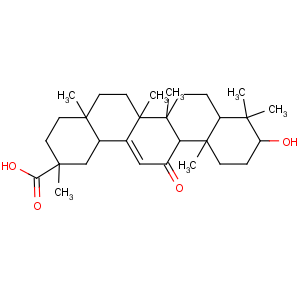
References of (2S,4aS,6aR,6aS,6bR,8aR,10S,12aS,14bR)-10-hydroxy-2,4a,6a,6b,9,9,
12a-heptamethyl-13-oxo-3,4,5,6,6a,7,8,8a,10,11,12,
14b-dodecahydro-1H-picene-2-carboxylic acid
Title: Enoxolone
CAS Registry Number: 471-53-4
CAS Name: (3b,20b)-3-Hydroxy-11-oxoolean-12-en-29-oic acid
Synonyms: 3b-hydroxy-11-oxoolean-12-en-30-oic acid; glycyrrhetic acid; 18b-glycyrrhetinic acid; uralenic acid
Trademarks: Arthrodont (Veyron-Froment); Biosone (Berk); P.O. 12 (Bioth?ax)
Molecular Formula: C30H46O4
Molecular Weight: 470.68
Percent Composition: C 76.55%, H 9.85%, O 13.60%
Literature References: From glycyrrhizic acid,
q.v. Structure: Ruzicka
et al., Helv. Chim. Acta 26, 2143, 2278 (1943). Stereochemistry: Beaton, Spring,
J. Chem. Soc. 1955, 3126. Prepn from shredded licorice root: Mer,
Am. Perfum. Aromat. 74(6), 39 (1959). Manuf:
GB 833184 (1960 to Carlo Erba). Identity with uralenic acid: Belous
et al., Zh. Obshch. Khim. 35, 401 (1965). Metabolism: Parke
et al., J. Pharm. Pharmacol. 15, 500 (1963). Mechanism of action: Helbing, Berntsen,
Pharm. Weekbl. 100, 1438 (1965).
Properties: Needles from alcohol + petr ether, mp 296°. [a]D21 +86° (alc); [a]D20 +145.5° (dioxane); [a]D20 +163° (chloroform). Freely sol in chloroform, dioxane. Soluble in alcohol, pyridine, acetic acid. Practically insol in petr ether.
Melting point: mp 296°
Optical Rotation: [a]D21 +86° (alc); [a]D20 +145.5° (dioxane); [a]D20 +163° (chloroform)
Derivative Type: 18a-Hydrogen Form
Properties: Platelets from dil alcohol, mp 335°. [a]D20 +140° (alcohol); [a]D +98° (c = 0.1 in chloroform). Sol in alcohol, dioxane, chloroform.
Melting point: mp 335°
Optical Rotation: [a]D20 +140° (alcohol); [a]D +98° (c = 0.1 in chloroform)
Therap-Cat: Anti-inflammatory (topical).
Keywords: Glucocorticoid.