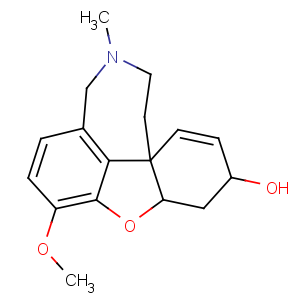
Title: Galantamine
CAS Registry Number: 357-70-0
CAS Name: (4a
S,6
R,8a
S)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6
H-benzofuro[3a,3,2-
ef][2]benzazepin-6-ol
Synonyms: galanthamine; lycoremine
Molecular Formula: C17H21NO3
Molecular Weight: 287.35
Percent Composition: C 71.06%, H 7.37%, N 4.87%, O 16.70%
Literature References: Selective acetylcholinesterase inhibitor. Isoln from Caucasian snowdrops,
Galanthus woronowii Vel.,
Amaryllidaceae: N. F. Proskurnina, A. P. Yakovleva,
J. Gen. Chem. USSR 22, 1899 (1952); from
Narcissus spp: Boit
et al., Ber. 90, 725, 2197 (1957). Structure work: S. Kobayashi
et al., Chem. Ind. (London) 1956, 177. Synthesis and stereochemistry: D. H. R. Barton, G. W. Kirby,
Proc. Chem. Soc. London 1960, 392;
eidem, J. Chem. Soc. 1962, 806. Asymmetric synthesis of isomers from L-tyrosine: K. Shimizu
et al., Heterocycles 8, 277 (1977). Biosynthesis studies: D. H. R. Barton
et al., J. Chem. Soc. 1963, 4545; W. D?bke,
Heterocycles 6, 551 (1977). Toxicology study: S. L. Friess
et al., Toxicol. Appl. Pharmacol. 3, 347 (1961). Clinical pharmacokinetics: U. Bickel
et al., Clin. Pharmacol. Ther. 50, 420 (1991). Review of pharmacology: A. L. Harvey,
Pharmacol. Ther. 68, 113-128 (1995); of clinical experience in Alzheimer's disease: R. Bullock,
Exp. Rev. Neurother. 4, 153-163 (2004); of synthesis and pharmacology: J. Marco-Contelles
et al., Chem. Rev. 106, 116-133 (2006).
Properties: Crystals from benzene, mp 126-127°. [a]D20 -118.8° (c = 1.378 in ethanol). Monoacidic base. Fairly sol in hot water; freely sol in alcohol, acetone, chloroform. Less sol in benzene, ether.
Melting point: mp 126-127°
Optical Rotation: [a]D20 -118.8° (c = 1.378 in ethanol)
Derivative Type: Hydrochloride
Molecular Formula: C17H21NO3.HCl
Molecular Weight: 323.81
Percent Composition: C 63.06%, H 6.85%, N 4.33%, O 14.82%, Cl 10.95%
Properties: Crystals from water, dec 256-257°. Sparingly sol in cold, more sol in hot water. Very sparingly sol in alcohol, acetone.
Derivative Type: Hydrobromide
CAS Registry Number: 1953-04-4
Trademarks: Nivalin (Waldheim); Razadyne (J & J); Reminyl (J & J)
Molecular Formula: C17H21NO3.HBr
Molecular Weight: 368.27
Percent Composition: C 55.44%, H 6.02%, N 3.80%, O 13.03%, Br 21.70%
Properties: Crystals from water, dec 246-247°. [a]D20 -93.1° (c = 0.1015 in 15 ml H2O). Sparingly sol in water. LD50 i.v. in mice (mg/kg): 5.2 ± 0.2 (Friess).
Optical Rotation: [a]D20 -93.1° (c = 0.1015 in 15 ml H2O)
Toxicity data: LD50 i.v. in mice (mg/kg): 5.2 ± 0.2 (Friess)
Therap-Cat: Cholinesterase inhibitor.
Keywords: Cholinesterase Inhibitor.