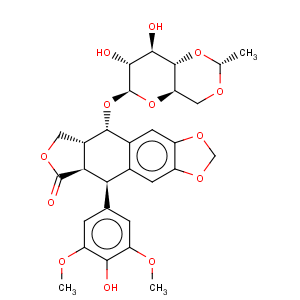
Title: Etoposide
CAS Registry Number: 33419-42-0
CAS Name: 9-[(4,6-
O-Ethylidene-b-D-glucopyranosyl)oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3¢,4¢:6,7]naphtho[2,3-
d]-1,3-dioxol-6(5a
H)-one
Synonyms: 4¢-demethylepipodophyllotoxin 9-[4,6-
O-ethylidene-b-D-glucopyranoside]; EPEG
Manufacturers' Codes: NSC-141540; VP-16-213
Trademarks: Lastet (Nippon Kayaku); Vepesid (BMS)
Molecular Formula: C29H32O13
Molecular Weight: 588.56
Percent Composition: C 59.18%, H 5.48%, O 35.34%
Literature References: DNA topoisomerase II inhibitor. Semi-synthetic deriv of podophyllotoxin, related structurally to teniposide,
q.q.v. Prepn: C. Keller-Juslén
et al., US 3524844 (1970 to Sandoz);
idem et al., J. Med. Chem. 14, 936 (1971). Teratogenicity and cytogenicity study: S. M. Sieber
et al., Teratology 18, 31 (1978). Review of pharmacokinetics and assay methods: P. I. Clark, M. L. Slevin,
Clin. Pharmacokinet. 12, 223-252 (1987). Comprehensive description: J. J. M. Holthuis
et al., Anal. Profiles Drug Subs. 18, 121-151 (1989). Review of pharmacology and clinical experience: J. D. Hainsworth, F. A. Greco,
Ann. Oncol. 6, 325-341 (1995); S. Joel,
Cancer Treat. Rev. 22, 179-221 (1996).
Properties: Crystals from methanol, mp 236-251°. [a]D20 -110.5° (c = 0.6 in chloroform). uv max (abs methanol): 283 nm (e 4245). pKa 9.8. Very sol in methanol, chloroform; slightly sol in ethanol; sparingly sol in water.
Melting point: mp 236-251°
pKa: pKa 9.8
Optical Rotation: [a]D20 -110.5° (c = 0.6 in chloroform)
Absorption maximum: uv max (abs methanol): 283 nm (e 4245)
Derivative Type: Phosphate
CAS Registry Number: 117091-64-2
Manufacturers' Codes: BMY-40481
Trademarks: Etopophos (BMS)
Molecular Formula: C29H33O16P
Molecular Weight: 668.54
Percent Composition: C 52.10%, H 4.98%, O 38.29%, P 4.63%
Literature References: Review: A. H. I. Witterland
et al., Pharm. World Sci. 18, 163-170 (1996).
Properties: Sol in water. Practically insol in organic solvents.
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Alkaloids/Natural Products; Podophyllum Derivatives; Topoisomerase II Inhibitor.