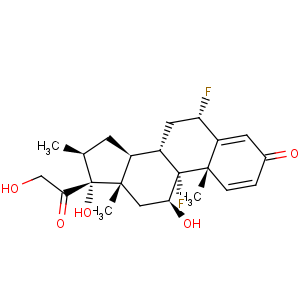
Title: Diflorasone
CAS Registry Number: 2557-49-5
CAS Name: (6a,11b,16b)-6,9-Difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione
Synonyms: 6a,9a-difluoro-16b-methyl-D1,4-pregnadiene-11b,17a,21-triol-3,20-dione; 6a,9a-difluoro-16b-methylprednisolone
Molecular Formula: C22H28F2O5
Molecular Weight: 410.45
Percent Composition: C 64.38%, H 6.88%, F 9.26%, O 19.49%
Literature References: The 16b-analog of flumethasone,
q.v. Prepn of free alcohol and 21-acetate:
GB 881334 (1961 to Pfizer),
C.A. 56, 15586c (1962);
GB 898293; F. H. Lincoln
et al., US 3557158 (1962, 1971 both to Upjohn);
GB 912015 (1962 to Merck & Co.). Prepn of the 17,21-diacetate: D. E. Ayer
et al., DE 2308731;
eidem, US 3980778 (1973, 1976 both to Upjohn). Proposed mechanism of action: S. Hammarstrom
et al., Science 197, 994 (1977). Pharmacology: S. Wickrema
et al., J. Invest. Dermatol. 71, 372 (1978). Clinical study: S. M. Bluefarb
et al., J. Int. Med. Res. 4, 454 (1976).
Derivative Type: Diacetate
CAS Registry Number: 33564-31-7
Manufacturers' Codes: U-34865
Trademarks: Dermaflor (Brocchieri); Diacort (Pfizer); Florone (Pfizer); Maxiflor (Allergan); Psorcon (Dermik); Soriflor (Nycomed)
Molecular Formula: C26H32F2O7
Molecular Weight: 494.52
Percent Composition: C 63.15%, H 6.52%, F 7.68%, O 22.65%
Properties: Crystals from ethyl acetate-Skellysolve C and acetone-methanol, mp 221-223° (dec). uv max (alc): 238 nm (e 17250). [a]D +61° (chloroform).
Melting point: mp 221-223° (dec)
Optical Rotation: [a]D +61° (chloroform)
Absorption maximum: uv max (alc): 238 nm (e 17250)
Therap-Cat: Anti-inflammatory (topical); glucocorticoid.
Keywords: Glucocorticoid.