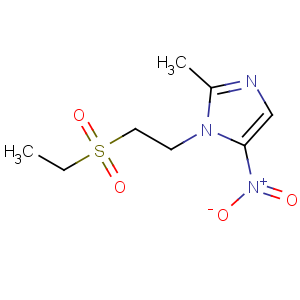
Title: Tinidazole
CAS Registry Number: 19387-91-8
CAS Name: 1-[2-(Ethylsulfonyl)ethyl]-2-methyl-5-nitro-1
H-imidazole
Synonyms: ethyl[2-(2-methyl-5-nitro-1-imidazolyl)ethyl]sulfone
Manufacturers' Codes: CP-12574
Trademarks: Fasigin (Pfizer); Fasigyn (Pfizer); Simplotan (Pfizer); Tindamax (Presutti); Tricolam (Pfizer); Trimonase (Mipharm)
Molecular Formula: C8H13N3O4S
Molecular Weight: 247.27
Percent Composition: C 38.86%, H 5.30%, N 16.99%, O 25.88%, S 12.97%
Literature References: Prepn: K. Butler,
US 3376311 (1968 to Pfizer); M. W. Miller
et al., J. Med. Chem. 13, 849 (1970). Series of articles on prepn, antiprotozoal activity and pharmacokinetics:
Antimicrob. Agents Chemother. 1969, 257-270. Review of antiprotozoal activity: P. R. Sawyer
et al., Drugs 11, 423-440 (1976); of antibacterial activity, pharmacology and therapeutic efficacy vs anaerobes: A. A. Carmine
et al., ibid. 24, 85-117 (1982). Symposium on clinical experience in anaerobic infection:
J. Antimicrob. Chemother. 10, Suppl. A, 1-184 (1982). Clinical trial in
Helicobacter pylori positive gastritis: G. Oderda
et al., Gut 33, 1328 (1992).
Properties: Colorless crystals from benzene, mp 127-128°. LD50 in mice (mg/kg): >3600 orally; >2000 i.p. (Miller).
Melting point: mp 127-128°
Toxicity data: LD50 in mice (mg/kg): >3600 orally; >2000 i.p. (Miller)
Therap-Cat: Antiprotozoal (Trichomonas, Giardia); antiamebic; antibacterial.
Keywords: Antiamebic; Antiprotozoal (Giardia); Antiprotozoal (Trichomonas).