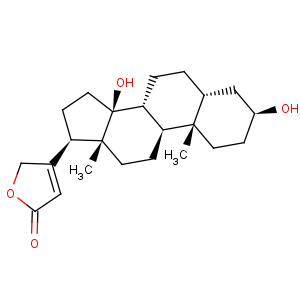
Title: Digitoxigenin
CAS Registry Number: 143-62-4
CAS Name: (3b,5b)-3,14-Dihydroxycard-20(22)-enolide
Synonyms: D20:22-3,14,21-trihydroxynorcholenic acid lactone; cerberigenin; echujetin; evonogenin
Trademarks: Thevetigenin
Molecular Formula: C23H34O4
Molecular Weight: 374.51
Percent Composition: C 73.76%, H 9.15%, O 17.09%
Literature References: The aglycon of digitoxin, thevetin, cerberin, echujin, evomonosid. Prepn by refluxing digitoxin in a mixture of water + alcohol + HCl: Cloetta,
Arch. Exp. Pathol. Pharmakol. 88, 113 (1920); Stoll, Kreiss,
Helv. Chim. Acta 17, 592 (1934). Structure: Jacobs, Hoffmann,
J. Biol. Chem. 67, 333 (1926); Jacobs, Gustus,
ibid. 78, 573;
79, 533 (1928);
82, 402 (1929); Jacobs, Elderfield,
ibid. 108, 497 (1935); Elderfield,
Chem. Rev. 17, 187 (1935). Stereochemistry: Meyer,
Helv. Chim. Acta 30, 1976 (1947). Synthesis: Danieli
et al., Tetrahedron 22, 3189 (1966); W. Fritsch
et al., Ann. 1974, 621; S. F. Donovan
et al., Tetrahedron Lett. 1979, 3287; R. Marinibettolo
et al., Can. J. Chem. 59, 1403 (1981); T. Milkova
et al., Tetrahedron Lett. 23, 413 (1982). Biosynthesis from neriifolin,
q.v.: A. Cruz
et al., J. Org. Chem. 42, 3580 (1977).
Properties: Stout prisms from 40% alc, mp 253°. [a]D17 +19.1° (c = 1.36 in methanol). Sol in alc, chloroform, acetone; slightly sol in ethyl acetate; very sparingly sol in ether, water.
Melting point: mp 253°
Optical Rotation: [a]D17 +19.1° (c = 1.36 in methanol)
Derivative Type: 3-Acetyldigitoxigenin
Molecular Formula: C25H36O5
Molecular Weight: 416.55
Percent Composition: C 72.08%, H 8.71%, O 19.20%
Properties: Hexagonal plates from acetone + ether, mp 222-227°. [a]D17 +21.4° (c = 1.02 in chloroform).
Melting point: mp 222-227°
Optical Rotation: [a]D17 +21.4° (c = 1.02 in chloroform)