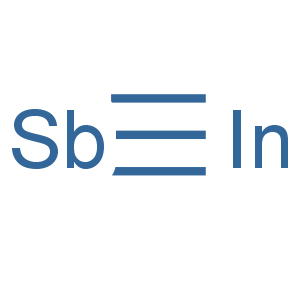Title: Indium Antimonide
CAS Registry Number: 1312-41-0
Molecular Formula: InSb
Molecular Weight: 236.58
Percent Composition: In 48.53%, Sb 51.47%
Literature References: Prepd by melting together stoichiometric amounts of indium and antimony in evacuated ampuls or in zone-refining apparatus: Kleppa,
J. Am. Chem. Soc. 77, 897 (1955); Harmon,
J. Electrochem. Soc. 103, 128 (1956).
Reviews: Welker, Weiss in
Solid State Physics vol. 3 (Academic Press, New York, 1956); Minden,
Sylvania Technol. 11, no. 1, 13-25 (Jan. 1958); Hulme, Mullin,
Solid State Electron. 5 (Pergamon Press, 1962) 211-247.
Properties: Crystals (zinc blende structure). mp 535°. d at mp 5.74 (solid); 6.48 (liq). Dielectric constant = 15.9. Energy gap at 25° = 0.18 ev. Hole mobility 1250 cm2/volt-sec. Electron mobility approx 80,000 cm2/volt-sec.
Melting point: mp 535°
Density: d at mp 5.74 (solid); 6.48 (liq)
Use: In semiconductor electronics. Grown p-n junctions have been made by doping a melt with an acceptor impurity such as zinc or cadmium, and dipping in an n-type crystal. Rate-grown junctions have also been made. Broad-area surface junctions have been produced by out-diffusing antimony in vacuum from the surface of an n-type crystal, producing a p-n junction just inside the surface. Also has photoconductive, photoelectromagnetic, and magnetoresistive properties. Useful as an infrared detector and filter, and in Hall effect devices.