References of Yohimban-16-carboxylicacid, 17-hydroxy-, methyl ester, (16b,17a,20a)-
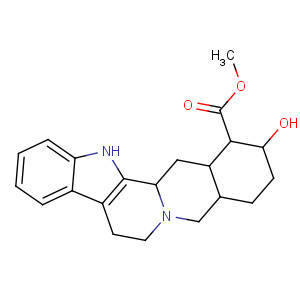
Title: a-Yohimbine
CAS Registry Number: 131-03-3
CAS Name: 17a-Hydroxy-20a-yohimban-16b-carboxylic acid methyl ester
Synonyms: corynanthidine; isoyohimbine; mesoyohimbine; rauwolscine
Molecular Formula: C21H26N2O3
Molecular Weight: 354.44
Percent Composition: C 71.16%, H 7.39%, N 7.90%, O 13.54%
Literature References: From bark of
Corynanthe johimbe K. Schum.,
Rubiaceae: Hahn, Brandenburg,
Ber. 60, 669 (1927); Wilbaut, van Gastel,
Rec. Trav. Chim. 54, 88 (1935); from
Rauwolfia canescens L.,
Apocynaceae: Mookerjee,
J. Indian Chem. Soc. 18, 33 (1941),
C.A. 35, 7967 (1941); Stoll
et al., Helv. Chim. Acta 38, 270 (1955). Identity with corynanthidine: Janot, Goutarel,
Bull. Soc. Chim. Fr. 1946, 535. Identity with isoyohimbine: Heinemann,
Ber. 60, 15 (1934). Identity with rauwolscine: Chatterjee
et al., Chem. Ind. (London) 1954, 491. Structure and stereochemistry: Le Hir
et al., Bull. Soc. Chim. Fr. 1953, 1027; Wenkert, Liu,
Experientia 11, 302 (1955); Aldrich
et al., J. Am. Chem. Soc. 81, 2481 (1959); Janot
et al., Bull. Soc. Chim. Fr. 1961, 637. Total synthesis: T?ke
et al., J. Org. Chem. 38, 2496 (1973).
Properties: Crystals, mp 243-244°. [a]D19 -18° (pyridine); [a]D19 -27° (abs alcohol). pKa 6.34. uv max (methanol): 227, 281 nm (log e 4.50, 3.93). Sol in warm methanol, ethanol; slightly sol in ether, benzene; practically insol in petr ether, water.
Melting point: mp 243-244°
pKa: pKa 6.34
Optical Rotation: [a]D19 -18° (pyridine); [a]D19 -27° (abs alcohol)
Absorption maximum: uv max (methanol): 227, 281 nm (log e 4.50, 3.93)
Derivative Type: Hydrochloride
Molecular Formula: C21H26N2O3.HCl
Molecular Weight: 390.90
Percent Composition: C 64.52%, H 6.96%, N 7.17%, O 12.28%, Cl 9.07%
Properties: Crystals, mp 288°. [a]D20 +55.5° (water).
Melting point: mp 288°
Optical Rotation: [a]D20 +55.5° (water)