Doxorubicin hydrochloride
-
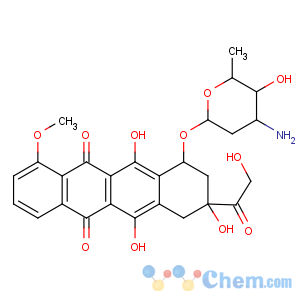
Molecular Structure
Detailed Description
Doxorubicin hydrochloride
Skype:Candice148722
Molecular Weight: 579.9802
CAS: 25316-40-9
EINECS . 246-818-3
Appearance: microstrip orange-red crystalline powder, no impurity
Content: ≥98%
PH: 4-5.5
Moisture: <4.0%
Impurities: 0.55
Uses: 1 Hodgkin's disease and lymphosarcoma, reticulum cell sarcoma.
2 undifferentiated small cell and non-small cell lung cancer, breast cancer.
3 acute lymphoblastic and myeloid leukemia.
4 osteosarcoma and soft tissue sarcoma.
5 ovarian cancer, testicular cancer, bladder cancer,. Wilms cancer, prostate cancer, thyroid cancer, neuroblastoma cancer, esophageal cancer, stomach cancer, liver cancer, cervical cancer and head and neck cancer. Multiple myeloma, pancreatic cancer, endometrial cancer and brain tumors can also be applied.
Packaging and storage: two layers of polyethylene bags plus 10 g / foil bag 100 g / aluminum foil bag. 1KG foil bags or cans.
Storage: sealed, refrigerated at 2-8 ° doxorubicin hydrochloride pharmacological effects of the active ingredient is passed through the nucleic acid binding interference for the synthesis of DNA and RNA, double-stranded DNA inserted into the base. In liposome-encapsulated doxorubicin and polyethylene glycol derivatives around the nucleus, forming doxorubicin liposomes.This technique is called "hidden technology" (stealthtechnology). Liposomal doxorubicin is bound to the hidden surface of the methoxy polyethylene glycol (MPEG). This polymer wrapped to protect the covert after liposome system from monocyte-macrophage phagocytosis, resulting in a significant increase in blood circulation time, may have to reduce doxorubicin cardiotoxicity, increased tumor target tissue absorption. Pharmacokinetics in vivo half-life of about 55 hours, can be measured after administration of low levels of Doxorubicinol (a metabolite of doxorubicin).After absorption, the product was distributed to the skin tissue. Slow plasma clearance at a dose of 20mg / m2, the average clearance 0.04lL / (h · m2). Indications before or can not tolerate the development of these AIDS-related Kaposi's sarcoma treatment of post-comprehensive treatment used. Dosage intravenous drip. Adult dose 20mg / m2, with 5% glucose solution 250ml diluted intravenous infusion of 30 to 60 minutes. Dilution at 2 ~ 8 ℃ cryopreservation stable 24 hours, but can not be mixed with other drugs, saline, antibiotics.
Drug corresponding effect:
1. Epirubicin can be used in combination with other anticancer drugs, but epirubicin dosage should be reduced. When combined with medication, should not be used in the same syringe.
2. Epirubicin injection should not be mixed with heparin, because both the chemical nature of the incompatibility occurs when precipitation reaction at a certain concentration.
Attentions: a disabled patient due to chemotherapy or radiotherapy and cause significant bone marrow suppression. 2. Have used large doses of anthracyclines (such as doxorubicin or daunorubicin) patients. 3. Recent or previous patient with a history of heart damage.
Price:Negotiable
zc02 at yccreate dot com

- Doxorubicin hydrochloride


 husiyu
husiyu



