D-Tartaric acid
-
- Product NameD-Tartaric acid
- CAS No.147-71-7
- Purity98%
- Min Quantity1000Kilograms
- Price~

 View Contact Detail
View Contact Detail
-
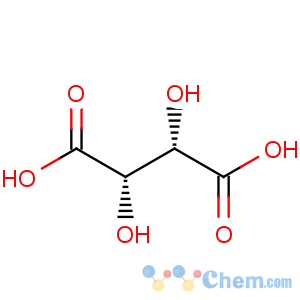 Molecular Structure
Molecular Structure

- D-Tartaric acid
Detailed Description
D-Tartaric acidEnglish Name: D-Tartaric acid
English alias: (S, S) -TARTARIC ACID; TARTARIC ACID; TARTARIC ACID, D- (-) - (+); TARTARIC ACID [DEXTRO; TARTARIC ACID UNNATURAL; TARTARIC (D-) ACID; UNNATURAL TARTARIC ACID; D-TA
Chinese alias: D- tartaric acid; D-2,3- two hydroxy butanedioic acid; [S- (R*, R*)]-2,3- two hydroxy butanedioic acid; D-2,3- two hydroxy succinic acid; two hydroxy succinic acid; grape; D- tartaric acid; d-tartaric acid; D-2,3- two hydroxy succinic acid; dextral grape acid; D (-) - tartaric acid
CAS number: 147-71-7
Molecular formula: C4H6O6
Molecular weight: 150.09
EINECS number: 205-695-6
Content: 99%
Packaging: 25 kg / cardboard drum
Chemical properties: tartaric acid has three stereoisomers: Stone spiral tartaric acid, L-tartaric acid and meso tartaric acid. A mixture of equal right-handed and left-handed form body rotation offset each other, known as the racemic tartaric acid. DL do not exist in nature, can be produced by chemical synthesis. A variety of tartaric acid is a colorless crystals, soluble in water.
Appearance: white crystalline powder
Use: tartaric acid and tannin combination, can be used as acid mordant dyes, also used in some developing and fixing operation photographic industry, its iron salt with the photosensitivity, so it can be used for the production of blueprint. Tartaric acid can complex with various metal ions, can be used for cleaning and polishing the surface of metal. Potassium sodium tartrate (Rochelle salt) can be prepared Fehling, also can be used in medicine as a laxative and diuretic, also used as intermediates of cinchophen. The crystals have piezoelectric properties, can be used in electronic industry.