You are here:
HomeProductsLevocetirizine Dihydrochloride 130018-87-0
Levocetirizine Dihydrochloride 130018-87-0
-
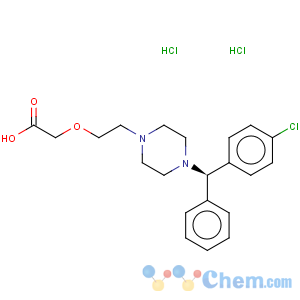
Molecular Structure
Detailed Description
Product Name: Levocetirizine Dihydrochloride
1. Basic Information:
Product Name Levocetirizine Dihydrochloride
Synonym Levocetirizine,Levocetirizine 2HCL,Levocetirizine DIHCL,Levocetirizine HCL,Xyzal,Xuzal,Zilola,2-(2-{4-[(R)-(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)acetic acid
CAS 130018-87-0
MF C21H27Cl3N2O3
MW 461.81
Assay 99%
Quality Standards USP/Pharma Grade
Appearance White powder
Package 5kg/Drum
Place of Origin China
Brand Name SMQ
Certification ISO9001, ISO14000, KOSHER
Model Number 130018-87-0
MOQ 1kg
Packaging Details 5kg/Drum,1kg/Foil bag
Delivery Time 1-2 days
Payment Terms T/T, Western union, Money gram
Supply Ability 50000 T/Month
2. Quick View:
Levocetirizine (as levocetirizine dihydrochloride) is a third-generation non-sedative antihistamine, developed from the second-generation antihistamine cetirizine. Chemically, levocetirizine is the active enantiomer of cetirizine. It is the R-enantiomer of the cetirizine racemate. Levocetirizine works by blocking histamine receptors. It does not prevent the actual release of histamine from mast cells, but prevents it binding to its receptors. This in turn prevents the release of other allergy chemicals and increased blood supply to the area, and provides relief from the typical symptoms of hay fever.
3. History and formulations:
(1) Levocetirizine was first launched in 2001 by Belgian pharmaceutical company UCB. It is sold under the brand name Xyzal in Australia, Austria, Croatia, Czech Republic, Finland, France, Ireland, Lithuania, Netherlands, Portugal, Romania, Taiwan, Turkey, The Philippines, United States, Serbia, Slovakia, Slovenia, South Africa and UK; Xuzal in Mexico; Xusal in Germany; Xazal in Spain; and Xozal in Greece. In Hungary it is marketed by Richter Gedeon under the Zilola brand name.
(2) In India, levocetirizine is marketed by GlaxoSmithKline and UCB under the brand name Vozet and Xyzal Respectively. On May 25, 2007, the United States Food and Drug Administration approved Xyzal, where it is co-marketed by Sanofi-Aventis. Torrent Pharma launched UVNIL in rural market of India. It is also available as LEZYNCET 5 mg tablets through Unichem in India. In India, generic name of Lev-Cit 5 mg is manufactured by VIP Pharmaceuticals. Also marketed in India by Croslands (Ranbaxy Laboratories Ltd.) under the brand name Teczine. In Brazil it is marketed under the brand name 'Zyxem' by Farmalab. It is marketed in Egypt by BORG Pharma under the brand name 'Xaltec', Allear by western pharmaceuticals and levcet by marcryl.
(3) In Pakistan levocetirizine was first launched in liquid formulation by Novartis Consumer Health Division by the name of T-Day Syrup. It is available as 5 mg-strength tablets and a 0.5 mg/mL oral solution. In Pakistan levocetirizine is available in liquid formulation as well with the name of OCITRA and T-Day 2.5 mg/5 mL. In Bangladesh levocetirizine is available in 5 mg tablet & 2.5 mg/5 mL oral liquid formulation with the brand name of Alcet marketed by Healthcare Pharmaceuticals and Seasonix marketed by Incepta Pharmaceuticals. In Nepal levocetirizine is available in 5 mg tablet with brand name of Curin manufactured by Beximco Pharma.

- Levocetirizine Dihydrochloride 130018-87-0




