Magnesium sulfate
-
- Product NameMagnesium sulfate
- CAS No.10034-99-8
- Purity99%
- Min Quantity1000Kilograms
- Price1~5

 View Contact Detail
View Contact Detail
-
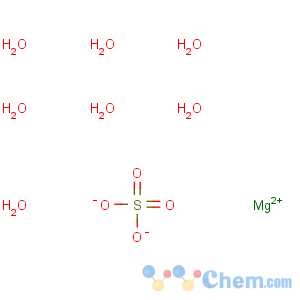 Molecular Structure
Molecular Structure
Detailed Description
Magnesium sulfate is an inorganic salt (chemical compound) containing magnesium, sulfur and oxygen, with the formula MgSO4. It is often encountered as the heptahydrate sulfate mineral epsomite (MgSO4·7H2O), commonly called Epsom salt. The monohydrate, MgSO4·H2O is found as the mineral kieserite. The overall global annual usage in the mid-1970s of the monohydrate was 2.3 million tons, of which the majority was used in agriculture.Anhydrous magnesium sulfate is used as a drying agent. The anhydrous form is hygroscopic (readily absorbs water from the air) and is therefore difficult to weigh accurately; the hydrate is often preferred when preparing solutions (for example, in medical preparations). Epsom salt has been traditionally used as a component of bath salts. Epsom salt can also be used as a beauty product. Athletes use it to soothe sore muscles, while gardeners use it to improve crops. It has a variety of other uses: for example, Epsom salt is also effective in the removal of splinters.