Succinic acid
-
- Product NameSuccinic acid
- CAS No.110-15-6
- Purity
- Min Quantity
- Price~

 View Contact Detail
View Contact Detail
-
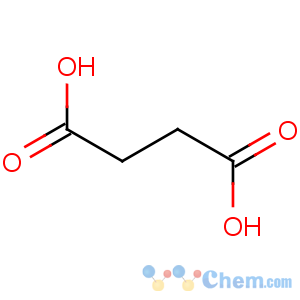 Molecular Structure
Molecular Structure
Detailed Description
Succinic acid is a colorless crystal; relative density 1.572 (25/4°C), melting point 188°C, decomposition at 235°C; distillation under reduced pressure can be sublimated; soluble in water, slightly soluble in ethanol, ethyl ether and acetone.In industry, succinic acid is usually obtained by catalytic reduction of butylenedioic acid, and succinic acid can also be prepared by hydrolysis of succinonitrile. In the laboratory, succinic acid can be reacted with two molecules of sodium diethyl malonate with iodine, followed by hydrolytic decarboxylation.
An important use of succinic acid is to prepare five-membered heterocyclic compounds. For example, succinic acid is rapidly dehydrated upon heating to form succinic anhydride, which is a furan ring compound. Succinic anhydride is an important raw material for the manufacture of drugs, dyes, and alkyd resins. Succinic anhydride is co-heated with ammonia to produce succinimide. Hydrogen on the imine group of the succinimide can be replaced by bromine to produce N-bromosuccinimide, an organic synthesis brominating agent and a mild oxidizing agent. Succinic acid has anticonvulsant, expectorant and diuretic properties in medicine. Diethyl succinate is an important intermediate for organic synthesis. Dibutyl succinate and dioctyl ester are plastic plasticizers. Diallyl succinate is copolymerized with 1,3-butadiene to produce an elastomer.