Tetracaine hydrochloride
-
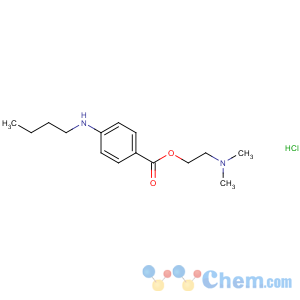
Molecular Structure
Detailed Description
Alias: Tetracaine hydrochloride,TetracaineHCL;pontocaine
CAS NO.: 136-47-0
EINECS: 205-248-5
MF: C15H25ClN2O2
MW: 300.82
Melting point: 149°C
Assay: 99%
Grade: Pharmaceutical Grade
Storage: Closed, confined and shading preservation
Usage: Local anesthetic; Mainly used in mucosa anesthetic. The function is stronger than Procaine and Lidocaine.
Catergory: API intermediates;local anesthetic;Sodium channel;Amines;Aromatics;Intermediates & Fine Chemicals;Pharmaceuticals;Pharmaceutical intermediate;pharmaceutical raw material;cytobiology;
Description:
Tetracaine HCl is synthesized from 4-butylaminobenzoic acid. The ethyl ester is formed through an acid-catalyzed esterification reaction. Base-catalyzed transesterification is achieved by boiling the ethyl ester of 4-butylaminobenzoic acid with excess 2-dimethylaminoethanol in the presence of a small amount of sodium ethoxide.
Application:
Tetracaine (INN, also known as amethocaine; trade name Pontocaine. Ametop and Dicaine) is a potent local anesthetic of the ester group. It is mainly used topically in ophthalmology and as an antipruritic, and it has been used in spinal anesthesia.
In biomedical research, tetracaine is used to alter the function of calcium release channels (ryanodine receptors) that control the release of calcium from intracellular stores. Tetracaine is an allosteric blocker of channel function. At low concentrations, tetracaine causes an initial inhibition of spontaneous calcium release events, while at high concentrations, tetracaine blocks release completely.

- Tetracaine hydrochloride




