You are here:
HomeProductsL-2-Aminobutyric acid, CAS 1492-24-6, Homoalaine
L-2-Aminobutyric acid, CAS 1492-24-6, Homoalaine
-
- Product NameL-2-Aminobutyric acid, CAS 1492-24-6, Homoalaine
- CAS No.1492-24-6
- Purity>99%
- Min Quantity300Metric Tons
- Price~

 View Contact Detail
View Contact Detail
-
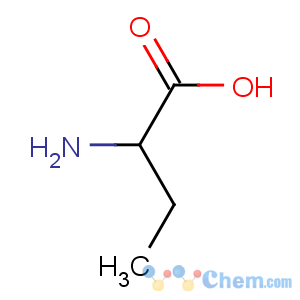
Molecular Structure
Detailed Description
What is L-2-Aminobutyric acid?
In biochemistry, L-2-Aminobutyric acid is also known as homoalanine. It is a non-proteinogenic alpha amino acid. The straight two carbon side chain is one carbon longer than alanine, hence the prefix homo-.
L-2-Aminobutyric acid is an isomer of the amino acid aminobutyric acid. It has two other isomers, gamma-aminobutyric acid (GABA) and beta-aminobutyric acid. In contrast, GABA is a neurotransmitter and not used in protein synthesis, and it is almost always referred to as GABA.
Application of L-2-Aminobutyric acid
L-2-Aminobutyric acid is an intermediate for medicine.
Synthesis of L-2-Aminobutyric acid
Homoalaine is biosynthesised by transaminating oxobutyrate, a metabolite in isoleucine biosynthesis. It is used by nonribosomal peptide synthases. One example of a nonribosomal peptide containing homoalanine is ophthalmic acid, which was first isolated from calf lens.
Ways to synthetize L-2-Aminobutyric acid
1.With a transamination reaction from l-threonine and l-aspartic acid as substrates in a whole cell biotransformation using recombinant Escherichia coli K12.
The cells contained the cloned genes tyrB, ilvA and alsS which respectively encode tyrosine aminotransferase of E. coli, threonine deaminase of E. coli and α-acetolactate synthase of B. subtilis 168. The 2-aminobutyric acid was produced by the action of the aminotransferase on 2-ketobutyrate and l-aspartate. The 2-ketobutyrate is generated in situ from l-threonine by the action of the deaminase, and the pyruvate by-product is eliminated by the acetolactate synthase. The concerted action of the three enzymes offers significant yield and purity advantages over the process using the transaminase alone with an eight to tenfold increase in the ratio of product to the major impurity.
2.From 2-oxobutyric acid and benzylamine with an enantiomeric excess higher than 99%.
Asymmetric synthesis of an unnatural amino acid was demonstrated by (t)-transaminasefrom Vibrio fluvialis JS17. The reaction showed severe product inhibition by benzaldehyde ,which was overcome by employing a biphasic reaction system to remove the inhibitory product from the aqueous phase. In a typical biphasic reaction (50 mM2-oxobutyric acid,70 mM benzylamine and 2.64 U/ml purified enzyme)using hexane as an extractant ,conversion of 2-oxobutyric acid reached 96% in 5h whereas only 39% conversion was obtained without the product extraction.
3.From DL-2-aminobutyric acid and L-dihydroxysuccinic acid or D-dihydroxysuccinic acid.
With DL-2-aminobutyric acid and L-dihydroxysuccinic acid or D-dihydroxysuccinic acid comprising reacting in inorganic acid. After cooling and refrigeration, separate out the solid compound and recrystalize it with water. After the aminolysis reaction of the recrystalized compound with ammonia solution, L-2-Aminobutyric acid is gained through the process of cooling, filtration and cleanse.
L-2-Aminobutyric acid prepared by this way is with high purity, high yield and much lower cost. It is feasible for large scale industrial production.

- L-2-Aminobutyric acid, CAS 1492-24-6, Homoalaine




