RHENIUM
-
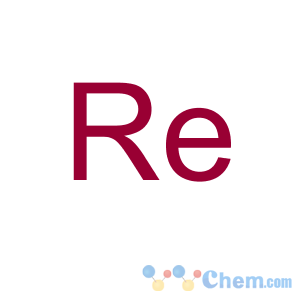
Molecular Structure
Other Information
- CAS Registry Number: 7440-15-5
- Transport: UN 3178 4
- Melting Point: 3180 °C(lit.)
- Flash Point: °C
- Boiling Point: 5596 °C(lit.)
- Density: 21,04 g/cm3
- Safety Statements: Low toxicity by ingestion. No reported cases of human toxicity. Experimentally, the Re3+ cation is more toxic than the ReO4− anion. Symptoms of Re3+ poisoning in rats are sedation, abdominal irritation, and death from cardiovascular collapse. Symptoms of ReO4− toxicity in rats include severe sedation and ataxia, tonic convulsions, and cardiovascular collapse. In cats, rhenium causes transient hypertension with tachycardia and transient auricular and ventricular fibrillations. In experimental animals, inhalation of rhenium dust causes pulmonary fibrosis.Radiation Hazard: Natural (63%) isotope 187Re, T1/2 = 4 × 1010 years, decays to stable 187Os by betas of less than 0.10 MeV. Flammable in the form of dust when exposed to heat or flame. Violent reaction with F2 @ 125°. Ignites in oxygen at 300°C. See also various rhenium compounds and RARE EARTHS.
- Hazard Symbols: Flammable in powder form.
- Flash Point: °C
- EINECS: 231-124-5 /231-714-2
- Molecular Weight: 186.207
- InchiKey: WUAPFZMCVAUBPE-UHFFFAOYSA-N
- InChI: InChI=1S/Re
- Risk Statements: S24/25:Avoidcontactwithskinandeyes.;
- Molecular Formula: Re

 View Contact Detail
View Contact Detail Molecular Structure
Molecular Structure