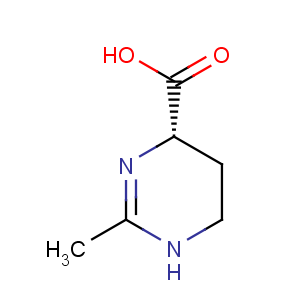
Title: Ectoine
CAS Registry Number: 96702-03-3
CAS Name: (4
S)-1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid
Synonyms: ectoin
Trademarks: RonaCare Ectoin (Merck KGaA)
Molecular Formula: C6H10N2O2
Molecular Weight: 142.16
Percent Composition: C 50.69%, H 7.09%, N 19.71%, O 22.51%
Literature References: Naturally occurring cyclic amino acid. Organic osmolyte; synthesized by microorganisms to maintain osmotic equilibrium in saline environments and to protect enzymes and whole cells from denaturation. Isoln from the halophilic, phototrophic bacterium,
Ectothiorhodospira halochloris, and characterization as a compatible solute: E. A. Galinski
et al., Eur. J. Biochem. 149, 135 (1985). Biosynthesis: P. Peters
et al., FEMS Microbiol. Lett. 71, 157 (1990). Calorimetric analysis of biosynthesis efficiency: T. Maskow, W. Babel,
Biochim. Biophys. Acta 1527, 4 (2001). Chromatographic determn: V. Riis
et al., Anal. Bioanal. Chem. 377, 203 (2003). Osmoprotection in
E. coli: M. Jebbar
et al., J. Bacteriol. 174, 5027 (1992). Mechanism of osmoprotection study:
idem et al., ibid. 187, 1293 (2005). Enzyme stabilization study: K. Lippert, E. A. Galinski,
Appl. Microbiol. Biotechnol. 37, 61 (1992).
In vitro prevention of UVA-induced skin damage: J. Buenger, H. Driller,
Skin Pharmacol. Physiol. 17, 232 (2004). Inhibition of b-amyloid peptide aggregation and neurotoxicity: M. Kanapathipillai
et al., FEBS Lett. 579, 4775 (2005).
Properties: Crystals from water-free methanol. mp ~280°. [a]D20 +140° (c = 1.0 in methanol). Soly at 4°: 6 mol/kg water. Non-ionic character at physiological pH.
Melting point: mp ~280°
Optical Rotation: [a]D20 +140° (c = 1.0 in methanol)
Use: Stabilizer in biotechnological processes; protects the taste of food during dehydration; moisturizer in cosmetics; protects skin against photoaging and formation of sunburn cells.