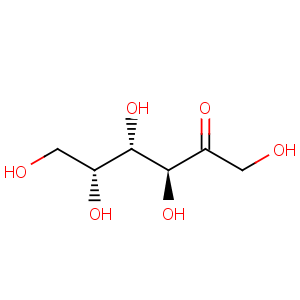
Title: D-Tagatose
CAS Registry Number: 87-81-0
Synonyms: D-
lyxo-Hexulose
Trademarks: Naturlose (Spherix)
Molecular Formula: C6H12O6
Molecular Weight: 180.16
Percent Composition: C 40.00%, H 6.71%, O 53.28%
Literature References: Uncommon, but naturally occurring ketohexose. Isoln from
Sterculia setigera gum: E. L. Hirst
et al., J. Chem. Soc. 1949, 3145. Prepn from D-galactose: C. A. L. De Bruyn, W. A. Van Ekenstein,
Rec. Trav. Chim. 16, 262 (1897); T. Reichstein, W. Bosshard,
Helv. Chim. Acta 17, 753 (1934); by biochemical oxidation of D-talitol: E. L. Totton, H. A. Lardy,
J. Am. Chem. Soc. 71, 3076 (1949); from lactose in heated milk: S. Adachi,
Nature 181, 840 (1958). Synthesis: M. L. Wolfrom, R. B. Bennett,
J. Org. Chem. 30, 1284 (1965); A. A. H. Al-Jobore
et al., Carbohydr. Res. 16, 474 (1971). Crystal structure: S. Takagi, R. D. Rosenstein,
ibid. 11, 156 (1969). Manuf process from whey: J. R. Beadle
et al., US 5002612 (1991 to Biospherics). Stability and sweetening properties in toothpaste: Y. Lu,
Int. J. Cosmet. Sci. 23, 175 (2001). Review of use as bulk sugar substitute: G. V. Levin
et al., Am. J. Clin. Nutr. 62, Suppl., 1161S-1168S (1995). Series of articles on toxicology and gastrointestinal tolerance:
Regul. Toxicol. Pharmacol. 29, S1-S93 (1999).
Properties: Crystals from aq ethanol, mp 131-133°. [a]D25 -5° (c = 1 in water). Sucrose-like taste, approx 92% as sweet as sucrose.
Melting point: mp 131-133°
Optical Rotation: [a]D25 -5° (c = 1 in water)
Derivative Type: L-Tagatose
CAS Registry Number: 17598-82-2
Literature References: Prepn: C. Glatthaar, T. Reichstein,
Helv. Chim. Acta 20, 1537 (1937). Stereoselective synthesis: T. Mukaiyama
et al., Chem. Lett. 1982, 1169.
Properties: Crystals, mp 134-135°. [a]D16 +1° (c = 2 in water).
Melting point: mp 134-135°
Optical Rotation: [a]D16 +1° (c = 2 in water)
Use: Non-nutritutive sweetener. Sweetening agent for pharmaceuticals and personal aid products.