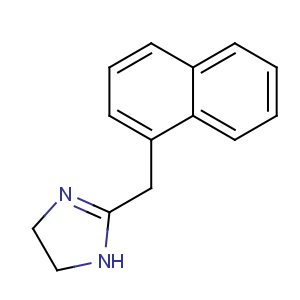
Title: Naphazoline
CAS Registry Number: 835-31-4
CAS Name: 4,5-Dihydro-2-(1-naphthalenylmethyl)-1
H-imidazole
Synonyms: 2-(1-naphthylmethyl)imidazoline
Molecular Formula: C14H14N2
Molecular Weight: 210.27
Percent Composition: C 79.97%, H 6.71%, N 13.32%
Literature References: a-Adrenergic agonist. Prepd by reacting the acetic acid anhydride of naphthoimidic acid with ethylenediamine: A. Sonn,
US 2161938 (1939 to Soc. Chem. Ind. Basle); by reacting naphthylthioacetamide with ethylenediamine:
DK 62889 (1944 to Lovens Kemiske),
C.A. 40, 4398 (1946). Toxicity data: J. Gylfe
et al., Fed. Proc. 9, 280 (1950). Comprehensive description: G. M. Wall,
Anal. Profiles Drug Subs. Excip. 21, 307-344 (1992).
Derivative Type: Hydrochloride
CAS Registry Number: 550-99-2
Trademarks: Ak-Con (Akorn); Albalon (Allergan); Clera (Person & Covey); Coldan; Iridina Due (Montefarmaco); Naphcon (Alcon); Niazol; Opcon (Bausch & Lomb); Privine (Novartis); Rhinantin; Rhinoperd; Sanorin; Sanorin-Spofa; Strictylon
Molecular Formula: C14H14N2.HCl
Molecular Weight: 246.74
Percent Composition: C 68.15%, H 6.13%, N 11.35%, Cl 14.37%
Properties: Bitter crystals, mp 255-260°. uv max (ethanol): 223, 270, 280, 287, 291 nm (E1%1cm 3622, 239, 286, 196, 198). pKa (25°C) 10.35 ±0.02, (35°C) 10.13 ±0.02, (45°C) 9.92 ±0.03. pKa (25°C) 10.35 ±0.02, (35°C) 10.13 ±0.02, (45°C) 9.92 ±0.03. Freely sol in water (40 g dissolve in 100 ml) and in alcohol. Slightly sol in chloroform. Insol in benzene, ether. A 1% aq soln has a pH of ~6.2. LD50 s.c. in rats: 385 mg/kg (Gylfe).
Melting point: mp 255-260°
pKa: pKa (25°C) 10.35 ±0.02, (35°C) 10.13 ±0.02, (45°C) 9.92 ±0.03; pKa (25°C) 10.35 ±0.02, (35°C) 10.13 ±0.02, (45°C) 9.92 ±0.03
Absorption maximum: uv max (ethanol): 223, 270, 280, 287, 291 nm (E1%1cm 3622, 239, 286, 196, 198)
Toxicity data: LD50 s.c. in rats: 385 mg/kg (Gylfe)
Therap-Cat: Adrenergic (vasoconstrictor); decongestant.
Keywords: a-Adrenergic Agonist; Decongestant.