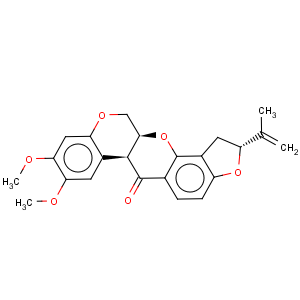
Title: Rotenone
CAS Registry Number: 83-79-4
CAS Name: [2
R-(2a,6aa,12aa)]-1,2,12,12a-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-[1]benzopyrano[3,4-
b]furo[2,3-
h][1]benzopyran-6(6a
H)-one
Trademarks: Canex (Mallinckrodt Vet.); Noxfire (AgrEvo)
Molecular Formula: C23H22O6
Molecular Weight: 394.42
Percent Composition: C 70.04%, H 5.62%, O 24.34%
Literature References: Principal insecticidal constituent of derris root, cubé, etc. Powerful inhibitor of mitochondrial electron transport. Isoln from
Lonchocarpus nicou (Aubl.) DC.,
Leguminosae: Geoffrey,
Ann. Inst. Colon. Marseille 2, 1 (1895). Review of structure: La Forge
et al., Chem. Rev. 12, 181 (1933); Butenandt, McCartney,
Ann. 494, 17 (1932); King,
Annu. Rep. Prog. Chem. 29, 186 (1932). Absolute configuration: Büchi
et al., J. Chem. Soc. 1961, 2843; Nakazaki, Arakawa,
Bull. Chem. Soc. Jpn. 34, 1246 (1961); Begley
et al., Chem. Commun. 1975, 850. NMR spectrum: Crombie, Lown,
ibid. 1962, 775. Total synthesis: Miyano
et al., Agric. Biol. Chem. 25, 673 (1961); Miyano,
J. Am. Chem. Soc. 87, 3958 (1965). Alternate synthesis: Crombie
et al., J. Chem. Soc. Perkin Trans. 1 1973, 1277,
eidem, Chem. Commun. 1979, 1142; I. Sasaki, K. Yamashita,
Agric. Biol. Chem. 43, 137 (1979). Synthesis of stereoisomers: Unai, Yamamoto,
ibid. 37, 897 (1973). Toxicology: Santi, Toth,
Farmaco Ed. Sci. 20, 270 (1965). Toxicity data: J.-I. Fukami
et al., Science 155, 713 (1967).
Review: H. Fukami, M. Nakajima in
Naturally Occurring Insecticides, M. Jacobson, D. G. Crosby, Eds. (Dekker, New York, 1971) pp 71-97; S. B. Soloway,
Environ. Health Perspect. 14, 109-117 (1976); T. J. Haley,
J. Environ. Pathol. Toxicol. 1, 315-337 (1978).
Properties: Orthorhombic, six-sided plates from trichloroethylene, mp 165-166° (dimorphic form, mp 185-186°). [a]D20 -228° (c = 2.22 in benzene). uv spectra: Büchi
et al., loc. cit. Practically insol in water. Sol in alcohol, acetone, carbon tetrachloride, chloroform, ether, and many other organic solvents. Dec upon exposure to light and air. Colorless solns in organic solvents oxidize upon exposure and become yellow, orange and then deep red and may deposit crystals of dehydrorotenone and rotenonone which are toxic to insects. LD50 i.p. in mice: 2.8 mg/kg (Fukami); in rats (mg/kg): 132 orally; 6 i.v. (Soloway).
Melting point: mp 165-166°; mp 185-186°
Optical Rotation: [a]D20 -228° (c = 2.22 in benzene)
Toxicity data: LD50 i.p. in mice: 2.8 mg/kg (Fukami); in rats (mg/kg): 132 orally; 6 i.v. (Soloway)
CAUTION: Potential symptoms of overexposure are irritation of eyes, skin, respiratory system; numbness of mucous membranes; nausea, vomiting, abdominal pain; muscle tremors, incontinence, clonic convulsions, stupor.
See NIOSH Pocket Guide to Chemical Hazards (DHHS/NIOSH 97-140, 1997) p 274. Inhalation may cause severe pulmonary irritation.
See Clinical Toxicology of Commercial Products, R. E. Gosselin
et al., Eds. (Williams & Wilkins, Baltimore, 5th ed., 1984) Section III, pp 366-368.
Use: Pesticide.
Therap-Cat-Vet: Ectoparasiticide.