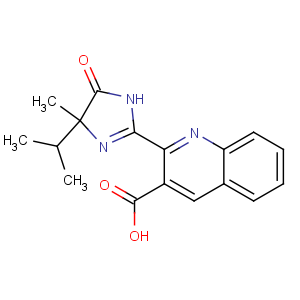
References of 2-(4-methyl-5-oxo-4-propan-2-yl-1H-imidazol-2-yl)quinoline-3-carboxylic
acid
Title: Imazaquin
CAS Registry Number: 81335-37-7
CAS Name: 2-[4,5-Dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1
H-imidazol-2-yl]-3-quinolinecarboxylic acid
Synonyms: 2-(5-isopropyl-5-methyl-4-oxo-2-imidazolin-2-yl)-3-quinolinecarboxylic acid
Manufacturers' Codes: AC-252214
Molecular Formula: C17H17N3O3
Molecular Weight: 311.34
Percent Composition: C 65.58%, H 5.50%, N 13.50%, O 15.42%
Literature References: Pre- and post-emergence imidazolinone herbicide especially for use in soybean crops. Prepn: M. Los,
EP 41623 (1981 to Am. Cyanamid),
C.A. 96, 199687q (1982). Alternate prepn: D. R. Maulding, R. F. Doehner, Jr.,
US 4459408 (1984 to Am. Cyanamid). Inhibition of branched-chain amino acid biosynthesis: D. L. Shaner
et al., Plant Physiol. 76, 545 (1984). Metabolism by plants: D. L. Shaner, P. A. Robson,
Weed Sci. 33, 469 (1985). Persistence in soil: G. Basham
et al., ibid. 35, 576 (1987). Herbicidal activity: M. W. Beale
et al., Proc. Annu. Meet. Northeast. Weed Sci. Soc. 38, 36 (1984); W. F. Congleton
et al., Weed Technol. 1, 186 (1987).
Properties: Crystals from hexane + ethyl acetate, mp 219-222° (dec). Slightly sol in some organic solvents. Soly in water at 25°: 60-120 ppm. LD50 orally in rats: 5000 mg/kg; LC50 (96 hr) in rainbow trout: 100 mg/l (Congleton).
Melting point: mp 219-222° (dec)
Toxicity data: LD50 orally in rats: 5000 mg/kg; LC50 (96 hr) in rainbow trout: 100 mg/l (Congleton)
Derivative Type: Ammonium salt
Trademarks: Scepter (BASF)
Molecular Formula: C17H20N4O3
Molecular Weight: 328.37
Percent Composition: C 62.18%, H 6.14%, N 17.06%, O 14.62%
Properties: Sol in water.
Use: Herbicide.