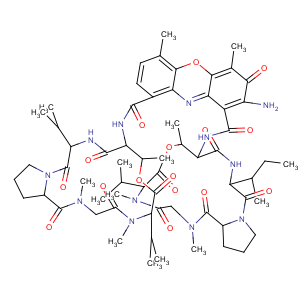
Title: Cactinomycin
CAS Registry Number: 8052-16-2
CAS Name: Actinomycin C
Manufacturers' Codes: HBF-386
Trademarks: Sanamycin (FBA Pharm)
Literature References: Antibiotic complex produced by
Streptomyces chrysomallus: Brockmann, Grubhofer,
Naturwissenschaften 36, 376 (1949);
37, 494 (1950);
Ber. 84, 260 (1951);
US 2953495 (1960 to Shenley Inds.); Lindenbein,
Arch. Mikrobiol. 17, 361 (1952). Mixture of actinomycins C1 (dactinomycin,
q.v.), C2 and C3, 10%, 45% and 45%, resp: Brockmann, Pfennig,
Naturwissenschaften 39, 429 (1952); Brockmann, Gr?ne,
ibid. 40, 222 (1953). Description of other actinomycins: Waksman
et al., Proc. Natl. Acad. Sci. USA 44, 602 (1958). Structures: Brockmann
et al., Angew. Chem. 68, 70 (1956); Brockmann, Boldt,
Naturwissenschaften 50, 19 (1963). Synthesis of actinomycin C3: Brockmann, Lackner,
ibid. 47, 230 (1960);
48, 555 (1961);
51, 407 (1964); Brockmann
et al., DE 1172680 (1964 to Bayer); Brockmann, Lackner,
Ber. 100, 353 (1967);
101, 1312 (1968). Synthesis of actinomycin C2: Brockmann, Lackner,
Tetrahedron Lett. 1964, 3517. Comprehensive review: H. Brockmann, in
Fortschr. Chem. Org. Naturst. 18, 1-54 (1960).
Properties: Alizarin-red hexagonal bipyramids from ethyl acetate, mp 252°. [a]D25 -325 to -349° (c = 0.25 in ethanol). Sparingly sol in water; moderately sol in ethanol; sol in chloroform, ethyl acetate, benzene, acetone.
Protect from light.
Melting point: mp 252°
Optical Rotation: [a]D25 -325 to -349° (c = 0.25 in ethanol)
Derivative Type: Actinomycin C2
Molecular Formula: C63H88N12O16
Molecular Weight: 1269.44
Percent Composition: C 59.61%, H 6.99%, N 13.24%, O 20.17%
Properties: Red bipyramids, prisms or needles from ethyl acetate, mp 237-239°. [a]D21 -325 ±10° (c = 0.23 in methanol). Abs max (methanol): 443 nm (e 25400).
Melting point: mp 237-239°
Optical Rotation: [a]D21 -325 ±10° (c = 0.23 in methanol)
Absorption maximum: Abs max (methanol): 443 nm (e 25400)
Derivative Type: Actinomycin C3
Molecular Formula: C64H90N12O16
Molecular Weight: 1283.47
Percent Composition: C 59.89%, H 7.07%, N 13.10%, O 19.95%
Properties: Red hexagonal bipyramids from ethyl acetate or methanol, dec 235°. [a]D17 -328° (c = 0.5 in ethanol). Absorption max (methanol): 443 nm (e 24100). Weak base.
Optical Rotation: [a]D17 -328° (c = 0.5 in ethanol)
Absorption maximum: Absorption max (methanol): 443 nm (e 24100)
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Antibiotics and Analogs; Actinomycins.