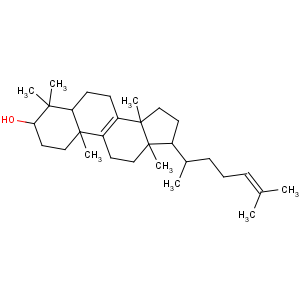
Title: Lanosterol
CAS Registry Number: 79-63-0
CAS Name: (3b)-Lanosta-8,24-dien-3-ol
Synonyms: kryptosterol
Molecular Formula: C30H50O
Molecular Weight: 426.72
Percent Composition: C 84.44%, H 11.81%, O 3.75%
Literature References: The core steroid from which all others are derived by biological modification. From wool fat of sheep: Windaus, Tschesche,
Z. Physiol. Chem. 190, 51 (1930). Identity with kryptosterol: Ruzicka
et al., Helv. Chim. Acta 28, 759 (1945). Structure: Voser
et al., ibid. 35, 2414 (1952); Barnes
et al., J. Chem. Soc. 1953, 571. Stereochemistry:
eidem, ibid. 1953, 576. Prepn from isocholesterol: Bloch, Urech,
Biochem. Prep. 6, 32 (1958). Prepn by cyclization of squalene: Cornforth
et al., Ciba Found. Symp. Terpenes Sterols 1958, 119; van Tamelen
et al., J. Am. Chem. Soc. 88, 4752 (1966). Mechanism of the squalene to lanosterol conversion:
eidem, ibid. 104, 6479, 6480 (1982).
Properties: Crystals, mp 138-140°. [a]D20 +62.0° (chloroform). .
Melting point: mp 138-140°
Optical Rotation: [a]D20 +62.0° (chloroform)
Derivative Type: Lanosteryl acetate
Properties: Crystals, mp 131.5-133°, [a]D20 +62.5° (c = 1.12 in chloroform).
Melting point: mp 131.5-133°
Optical Rotation: [a]D20 +62.5° (c = 1.12 in chloroform)