Title: Hydroxylamine
CAS Registry Number: 7803-49-8
Molecular Formula: H3NO
Molecular Weight: 33.03
Percent Composition: H 9.15%, N 42.41%, O 48.44%
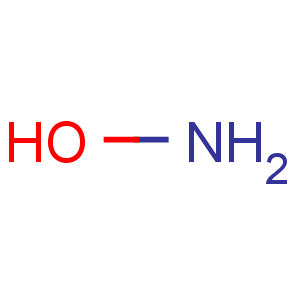
Line Formula: NH2OH
Literature References: Prepn as the hydrochloride: W. L. Semon,
Org. Synth. coll. vol. I, 318 (1932). Prepn: Hurd,
Inorg. Synth. 1, 87 (1939); Benson
et al., J. Am. Chem. Soc. 78, 4202 (1956). Crystal structure: Meyers, Lipscomb,
Acta Crystallogr. 5, 583 (1955). Toxicity data: Riemann,
Acta Pharmacol. Toxicol. 6, 285 (1950); R. P. Smith, W. R. Layne,
J. Pharmacol. Exp. Ther. 165, 30 (1969). Mutagenic action: Phillips, Brown in
Prog. Nucleic Acid Res. Mol. Biol. 7, 349-368 (1967).
Reviews: Mason, "Hydroxylamine" in
Mellor's vol. VIII, supplement 2,
Nitrogen (part 2), 115-157 (1967); Jones in
Comprehensive Inorganic Chemistry vol. 2, J. C. Bailar, Jr.
et al., Eds. (Pergamon Press, Oxford, 1973) pp 265-276.
Properties: Unstable, large white flakes or needles, mp 33°, bp22 58°. d40 1.2255; d440 1.204. pK (20°) 7.97. Very sol in water, liq ammonia and methanol. The soly in the higher alcohols decreases with increasing mol wt. Sparingly sol in ether, benzene, carbon disulfide, chloroform. Very hygroscopic. Dec by hot water. Undergoes rapid decompn at room temps esp in the presence of atm moisture and CO2. Detonates in test tube heated with flame. LD50 i.p. in mice: 1.83 mmol/kg (Smith, Layne).
Melting point: mp 33°
Boiling point: bp22 58°
pKa: pK (20°) 7.97
Density: d40 1.2255; d440 1.204
Toxicity data: LD50 i.p. in mice: 1.83 mmol/kg (Smith, Layne)
Derivative Type: Hydrochloride
CAS Registry Number: 5470-11-1
Synonyms: Oxammonium hydrochloride
Molecular Formula: H3NO.HCl
Molecular Weight: 69.49
Percent Composition: H 5.80%, N 20.16%, O 23.02%, Cl 51.02%
Properties: Monoclinic columnar crystals; slowly dec when moist. d17 1.67. mp about 151°. One gram dissolves in about 1 ml water (83 g in 100 ml water at 17°); 19 ml alcohol; 8 ml methanol. Sol in glycerol, propylene glycol. Insol in cold ether. pH of 0.2 molar aq soln 3.2. Keep well closed. LD50 orally in mice: 408 mg/kg (Riemann).
Melting point: mp about 151°
Density: d17 1.67
Toxicity data: LD50 orally in mice: 408 mg/kg (Riemann)
Derivative Type: Sulfate
CAS Registry Number: 10039-54-0
Synonyms: Oxammonium sulfate
Molecular Formula: (H3NO)2.H2SO4
Molecular Weight: 164.14
Percent Composition: H 4.91%, N 17.07%, O 58.48%, S 19.54%
Properties: Crystals, mp about 170°. Freely sol in water.
Melting point: mp about 170°
CAUTION: Skin irritant. May cause methemoglobinemia, sulfhemoglobinemia, cyanosis, convulsions, hypotension and coma.
See Clinical Toxicology of Commercial Products, R. E. Gosselin
et al., Eds. (Williams & Wilkins, Baltimore, 5th ed., 1984) Section II, p 117.
Use: As reducing agent in photography; in synthetic and analytical chemistry; to purify aldehydes and ketones. As antioxidant for fatty acids and soaps. As dehairing agent for hides.