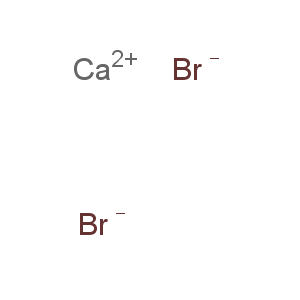Title: Calcium Bromide
CAS Registry Number: 7789-41-5
Molecular Formula: Br2Ca
Molecular Weight: 199.89
Percent Composition: Br 79.95%, Ca 20.05%
Line Formula: CaBr2
Literature References: Prepn:
Gmelins, Calcium (8th ed.)
28B, 100-102, 584-599 (1958). The N.F. article is a hydrated salt, contg not less than 84% and not more than 94% CaBr2.
Properties: Odorless, deliquesc granules or rhombic crystals; sharp, saline taste. Becomes yellow on long exposure to air. When anhydr mp 730°; d425 3.353. When strongly heated in air, becomes alkaline due to loss of bromine and formation of lime. Very sol in water, methanol, ethanol; sol in acetone; practically insol in dioxane, chloroform, ether. The aq soln is neutral or only slightly alkaline to litmus.
Keep well closed.
Melting point: mp 730°
Density: d425 3.353
Use: In photography for making dry plates and light-sensitive papers; manuf mineral waters, NH4Br, fire-extinguishing compositions.
Therap-Cat: Sedative, anticonvulsant.
Therap-Cat-Vet: Has been used in hypocalcemic states such as canine eclampsia.
Keywords: Anticonvulsant; Sedative/Hypnotic; Bromides.