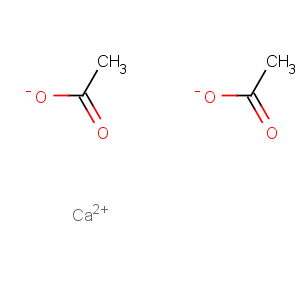
Title: Calcium Acetate
CAS Registry Number: 62-54-4
CAS Name: Acetic acid calcium salt
Trademarks: Phos-ex (Vitaline); PhosLo (Nabi)
Molecular Formula: C4H6CaO4
Molecular Weight: 158.17
Percent Composition: C 30.37%, H 3.82%, Ca 25.34%, O 40.46%
Line Formula: Ca(CH3COO)2
Literature References: The technical product was originally known as
brown acetate of lime or
gray acetate of lime. Prepn and analysis of acetate of lime: Stillwell, Gladding,
J. Am. Chem. Soc. 4, 94 (1882); T. S. Gladding,
Ind. Eng. Chem. 1, 250 (1909). Distillation to produce acetone: E. G. R. Ardagh
et al., ibid. 16, 1133 (1924). Electrolytic prepn: H. Schmidt,
Z. Anorg. Allg. Chem. 270, 188 (1952). Analysis of hydrated forms: J. Panzer,
J. Chem. Eng. Data 7, 140 (1962). Toxicity study: Smyth
et al., Am. Ind. Hyg. Assoc. J. 30, 470 (1969). Clinical evaluation in hyperphosphatemia: W. Y. Qunibi
et al., Kidney Int. 65, 1914 (2004).
Properties: Very hygroscopic, rod-shaped crystals. On heating above 160° dec to acetone and CaCO3. d 1.50. Sol in water; slightly sol in methanol. Practically insol in ethanol, acetone, benzene.
Keep well closed.
Density: d 1.50
Derivative Type: Monohydrate
CAS Registry Number: 5743-26-0
Properties: Needles, granules or powder. Does not lose all its water below 150°. Sol in water; slightly sol in alcohol. pH of 0.2 molar aq soln 7.6. LD50 orally in rats: 4.28 g/kg (Smyth).
Toxicity data: LD50 orally in rats: 4.28 g/kg (Smyth)
Use: Manuf of acetic acid, acetone; in dyeing, tanning, and curing skins; in lubricants; as food stabilizer; as corrosion inhibitor.
Therap-Cat: Antihyperphosphatemic.
Keywords: Antihyperphosphatemic.