Title: Sodium Tripolyphosphate
CAS Registry Number: 7758-29-4
CAS Name: Triphosphoric acid pentasodium salt
Synonyms: sodium triphosphate; tripolyphosphate; STPP; poly; pentasodium triphosphate
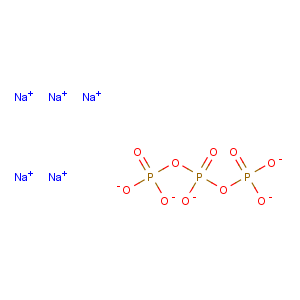
Molecular Formula: Na5O10P3
Molecular Weight: 367.86
Percent Composition: Na 31.25%, O 43.49%, P 25.26%
Line Formula: Na5P3O10
Literature References: Prepd by molecular dehydration of mono- and disodium phosphates: Audrieth, Bell,
Inorg. Synth. 3, 85 (1950). Toxicity study: H. F. Smyth
et al., Am. Ind. Hyg. Assoc. J. 30, 470 (1969).
Properties: Slightly hygroscopic granules. Soly in water (g/100 ml) at 25°: 20; at 100°: 86.5. pH of 1% soln at 25° = 9.7-9.8. Its properties are between those of tetrasodium pyrophosphate and sodium metaphosphate. Stability: With prolonged heating of STPP solns, it tends to revert to the orthophosphate. More stable than the higher (i.e., meta-) phosphates, but less stable than tetrasodium pyrophosphate. LD50 orally in rats: 6.50 g/kg (Smyth).
Toxicity data: LD50 orally in rats: 6.50 g/kg (Smyth)
CAUTION: Moderately irritating to skin, mucous membranes. Ingestion can cause violent purging.
Use: In water softening (calcium and magnesium hardness is sequestered from soln without precipitation); peptizing agent, emulsifier and dispersing agent, ingredient of cleansers in drilling fluids to control mud viscosity in oil fields; as preservative, sequestrant and texturizer in foods.